Supramolecular chemists find themselves working evermore at the intersection of chemistry and biology. Indeed, a key aim in contemporary supramolecular chemistry is to address salient problems associated with malfunctioning biological macromolecules (biomacromolecules). Hence, this volume provides a timely overview on the fundamental supramolecular chemistry aspects of selectively binding to, and inhibiting the function of, (deoxy)ribonucleic acids (DNA/RNA) and proteins—Companion volume 5 explores the medicinal aspects and ramifications of such interactions in greater detail. Furthermore, supramolecular chemists are traversing a two-way street (i.e., in conjunction to developing systems that interact with biomacromolecules), the field also takes much inspiration from the elegant structure, assembly states, and function of nature’s highly evolved biomacromolecules. As such, this volume also provides a series of reviews on self-assemblies and nanomaterials inspired from a signature biological context. These articles serve to illustrate how supramolecular chemists are leading the charge in harnessing the programmable conformations, self-assembly, and molecular recognition capacities of biomacromolecules or their synthetic congeners for applications ranging from materials to medicine.
Publications
2017
Host–guest complexes are emerging as powerful components in functional systems with applications ranging from materials to biomedicine. In particular, CB7 based host–guest complexes have received much attention for the controlled release of drugs due to the remarkable ability of CB7 toward binding input molecules in water with high affinity leading to displacement of CB7 from included pharmacophores (or from drug loaded porous particles). However, the release of bound guests from CB7 in response to endogenous biological molecules remains limited since the input biomolecule needs to have the appropriate chemical structure to bind tightly into the CB7 cavity. Herein we describe a synthetic transducer based on self-assembling DNA–small molecule chimeras (DCs) that is capable of converting a chosen biological input, adenosine triphosphate (ATP; that does not directly bind to the CB7 host), into functional displacement of a protein inhibitor that is bound within the CB7 host. Our system—which features the first example of a covalent CB-DNA conjugate—is highly modular and can be adapted to enable responsiveness to other biologically/clinically relevant stimuli via its split DNA aptamer architecture.

2016
A naphthalimide based fluorescent sensor displaying a significant increase in emission upon binding CB[7] with notable pH stability was developed and utilized in a surface-bound displacement assay for the rapid detection of CB[7] encapsulation of therapeutically relevant drug classes. Previously unknown binders with moderate to strong affinities were discovered.
2015
Epigenetic regulation of gene expression is critical to phenotypic maintenance and transition of human breast cancer cells. HOX antisense intergenic RNA (HOTAIR) is a long intergenic non-coding RNA that epigenetically represses gene expression via recruitment of enhancer of zeste homolog 2 (EZH2), a histone methyltransferase. Elevated expression of HOTAIR promotes progression of breast cancer. In the current study we examined the expression and function of HOTAIR in MCF-7-TNR cells, a derivative of the luminal-like breast cancer cell line MCF-7 that acquired resistance to TNF-α-induced cell death. The expression of HOTAIR, markers of the luminal-like and basal-like subtypes, and growth were compared between MCF-7 and MCF-7-TNR cells. These variables were further assessed upon inhibition of HOTAIR, EZH2, p38 MAPK, and SRC kinase in MCF-7-TNR cells. When compared with MCF-7 cells, MCF-7-TNR cells exhibited an increase in the expression of HOTAIR, which correlated with characteristics of a luminal-like to basal-like transition as evidenced by dysregulated gene expression and accelerated growth. MCF-7-TNR cells exhibited reduced suppressive histone H3 lysine27 trimethylation on the HOTAIR promoter. Inhibition of HOTAIR and EZH2 attenuated the luminal-like to basal-like transition in terms of gene expression and growth in MCF-7-TNR cells. Inhibition of p38 and SRC diminished HOTAIR expression and the basal-like phenotype in MCF-7-TNR cells. HOTAIR was robustly expressed in the native basal-like breast cancer cells and inhibition of HOTAIR reduced the basal-like gene expression and growth. Our findings suggest HOTAIR-mediated regulation of gene expression and growth associated with the basal-like phenotype of breast cancer cells. © 2014 Wiley Periodicals, Inc.
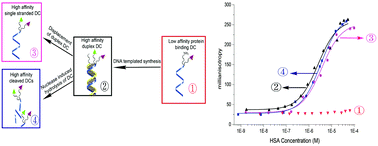
Herein we disclose the development of two complementary single stranded DNA-small molecule chimeras (DCs) that by themselves only bind weakly to a protein target (human serum albumin; HSA). However, upon self-assembly, the DC duplex facilitates a ligand migration reaction leading to a covalently fastened high-affinity, bidentate, protein-binder that resides at the terminus of only one of the DC strands. Due to this specific localization, the bidentate projection remains intact—and thus the system continues to strongly bind HSA—even under conditions that denature and degrade the DNA scaffolds.
In order to tackle the issue of systemic toxicity in chemotherapy, there is a need to develop novel mechanisms for the activation of protein inhibitors using biomarkers overexpressed in cancer cells. Many current strategies focus on using cancer associated enzymes as a triggering agent for prodrugs. Herein, we detail an alternative approach that harnesses a microRNA (miR-21) that is overexpressed in cancers as the trigger that activates an inhibitor of human carbonic anhydrase-II (hCA-II). Specifically, we have developed a DNA-small molecule chimera (DC) composed of an hCA-II binding lithocholic acid amide (LAA) headgroup that can transition from a rigid duplex state (that does not bind appreciably to hCA) to a single-stranded conformation via a miR-21 trigger. The activated single-stranded DC can project the LAA headgroup into the hCA-II active site and is a robust hCA-II inhibitor (Ki of 3.12 μM). This work may spur research into developing new classes of cancer selective protein inhibitors.

In materials, energy can propagate by means of two limiting regimes: diffusive and ballistic. Ballistic energy transport can be fast and efficient and often occurs with a constant speed. Using two-dimensional infrared spectroscopy methods, we discovered ballistic energy transport via individual polyethylene chains with a remarkably high speed of 1440 m/s and the mean free path length of 14.6 Å in solution at room temperature. Whereas the transport via the chains occurs ballistically, the mechanism switches to diffusive with the effective transport speed of 130 m/s at the end-groups attached to the chains. A unifying model of the transport in molecules is presented with clear time separation and additivity among the transport along oligomeric fragments, which occurs ballistically, and the transport within the disordered fragments, occurring diffusively. The results open new avenues for making novel elements for molecular electronics, including ultrafast energy transporters, controlled chemical reactors, and sub-wavelength quantum nanoseparators.
The development of methods to grow well-ordered chromophore thin films on solid substrates is of importance because such surface-associated arrays have potential applications in the generation of functional electronic and optical materials and devices. In this article, we demonstrate a straightforward layer-by-layer (LBL) supramolecular deposition strategy to prepare numerous layers (up to 19) of functionalized perylene diimide (PDI) chromophores built upon a covalent scaffolding multivalent porphyrin monolayer. Our thin film formation strategy employs water as the immersion solvent and exploits the β-cyclodextrin–adamantane host–guest couple in addition to PDI based aromatic stacking. Within the resultant film the porphyrin scaffold is oriented close to parallel to the glass substrate while the PDI chromophores are aligned closer to the surface normal. Together, the porphyrin monolayer and the multi-PDI layers exhibit a large absorption bandwidth in the visible spectrum. Importantly, because a self-assembly strategy was utilized, when a single monolayer of PDI is deposited on the porphyrin scaffolding layer, this PDI monolayer can be readily disassembled by washing with DMF leading to the regeneration of the porphyrin monolayer. The PDI thin film can subsequently be regrown from the regenerated porphyrin surface. The reported LBL strategy will be of broad interest for researchers developing well-organized chromophoric films and materials due to its simplicity as well as the added advantage of being performed in sustainable and cost-effective aqueous media.

Intramolecular transport of vibrational energy in two series of oligomers featuring alkane chains of various length was studied by relaxation-assisted two-dimensional infrared spectroscopy. The transport was initiated by exciting various end-group modes (tags) such as different modes of the azido (ν(N≡N) and ν(N═N)), carboxylic acid (ν(C═O)), and succinimide ester (νas(C═O)) with short mid-IR laser pulses. It is shown that the transport via alkane chains is ballistic and the transport speed is dependent on the type of the tag mode that initiates the transport. The transport speed of 8.0 Å/ps was observed when initiated by either ν(C═O) or νas(C═O). When initiated by ν(N≡N) and ν(N═N), the transport speed of 14.4 ± 2 and 11 ± 4 Å/ps was observed. Analysis of the vibrational relaxation channels of different tags, combined with the results for the group velocity evaluation, permits identification of the chain bands predominantly contributing to the transport for different cases of the transport initiation. For the transport initiated by ν(N≡N) the CH2 twisting and wagging chain bands were identified as the major energy transport channels. For the transport initiated by ν(C═O), the C–C stretching and CH2 rocking chain bands served as major energy transporters. The transport initiated by ν(N═N) results in direct formation of the wave packet within the CH2 twisting and wagging chain bands. These developments can aid in designing molecular systems featuring faster and more controllable energy transport in molecules.


![Graphical abstract: A naphthalimide derived fluorescent sensor for solid-phase screening of cucurbit[7]uril–guest interactions](https://pubs.rsc.org/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=C5CC08350H&imageInfo.ImageIdentifier.Year=2016)