Research
Research in Wang Lab at Tulane has been focused on the intersections of noncoding RNAs, vascular biology and retinal degeneration. Our research is highly relevant to degenerative retinal diseases including age-related macular degeneration (AMD), as well as cardiovascular diseases.
AMD is the leading cause of blindness in the elderly, affecting ~8.7% percent of the worldwide population. It has both wet and dry forms. Late-stage dry AMD is characterized by degeneration and loss of retinal pigment epithelial (RPE) cells, while wet AMD is characterized by choroidal neovascularization. Our lab was among the first labs to study the role of microRNAs (including miR-126, miR-23/27, miR-24) in ocular angiogenesis and in wet AMD mouse models. We have also shown miR-143/145 regulates intraocular pressure (IOP), which has implications in glaucoma. We were pioneering the efforts to study the function of long noncoding RNAs (lncRNAs) in human angiogenesis and established lncEGFL7OS as a human/primate-specific, endothelial cell-enriched lncRNA that is critical for angiogenesis through regulating MAX transcription factor activity at the EGFL7/miR-126 locus.
RPE cell death and senescence could significantly contribute to AMD, although the underlying mechanism remains unclear. Our lab has shown that necrosis but not apoptosis is a major type of RPE cell death in response to oxidative stress. We have conducted a chemical screening of a library with 1840 FDA-approved drugs and natural products and identified several compounds that can prevent oxidative stress-induced RPE cell death. These FDA-approved drugs could be repurposed novel anti-AMD drugs.
Cellular senescence is a phenomenon originally defined as stable cell cycle arrest in response to various stresses. Accumulation of senescence cells could drive aging and age-related diseases. Using genetic mouse model, our lab has determined the important function of mitochondria dynamics in senescence and found that mitochondrial hyperfusion drives accelerated RPE senescence through regulating AMPK/mTOR signaling.
Showing below are examples of some projects in the lab.
RPE Cell Death
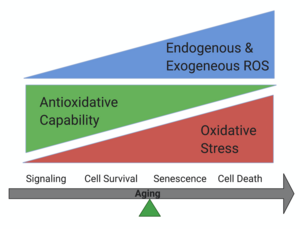
RPE cells are a monolayer of cell locate in between the neural retina and the choroid. RPE cell degeneration and death induced by oxidative stress is believed to contribute to the progress of AMD.
There are many ways a cell can die, including apoptosis, necroptosis, ferroptosis, pyroptosis and et al. However, how RPE cell die in AMD is still controversial. Previously, we found that RPE cells die through necroptosis in NaIO3 injected dry AMD mouse model, also observed the activation of RIPK3 (a necroptosis marker) in RIPK3-GFP transgenetic mice. In our recent publication, we once again confirmed the important role of necroptosis in RPE cell death, while ferroptosis is also involved in the process. We also found that necrosome is activated in ferroptotic RPE cells.In the future, we'll test more potential crosstalk among different cell death pathways, and we'll find out more potential. therapeutic targets for AMD.
Cellular Senescence
 Enter a detailed description of this area of your research. Image size is 300 x 200
Enter a detailed description of this area of your research. Image size is 300 x 200
Lorem ipsum dolor sit amet. Sed ipsam veritatis et voluptas impedit est velit sint in aspernatur magnam et sapiente ipsam est autem voluptates. Non maxime sunt et impedit quia et dolores culpa et accusamus nemo. Eum ratione voluptatibus est numquam iste id dolores excepturi ut explicabo magnam aut consectetur minima.
Qui reiciendis iste non enim autem eos ipsa galisum 33 dignissimos enim et delectus quidem? Et praesentium voluptate vel atque sint ut corrupti quae.
Noncoding RNAs in Ocular Fibrosis
 Enter a detailed description of this area of your research. Image size is 300 x 200
Enter a detailed description of this area of your research. Image size is 300 x 200
Subretinal fibrosis underlies the end-stage pathogenesis of several retinal diseases including age-related macular degeneration (AMD) and proliferative vitreoretinopathy (PVR). The cellular and molecular mechanism of subretinal fibrosis is still unclear, and the clinical treatment for this condition is very limited. The proposal will define the function, identity, and origin of myofibroblasts in subretinal fibrosis; and determine the therapeutic potential of miR-24 in subretinal fibrosis.
Laboratory Collaborations
(Name of lab) current collaborators include: list names here. If you've added collaborators to your team page, link to their profiles. For example: Dr. Layla Cronin, Boston Children's Hospital.
