Research
Research Area 1: Biomechanics of Myeloid, Endothelial, and Cancer Cells
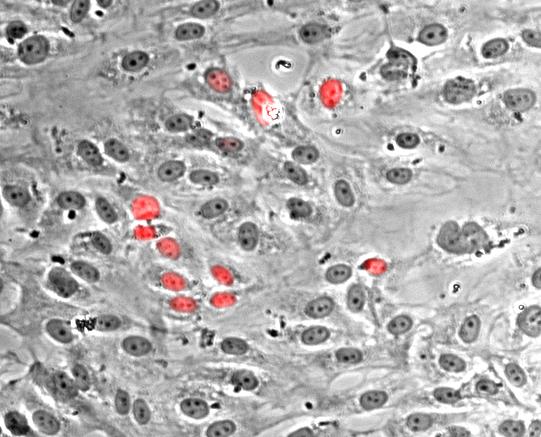 Nuclei of proliferating endothelial cells stained with Hoechst dye (red).
Nuclei of proliferating endothelial cells stained with Hoechst dye (red).
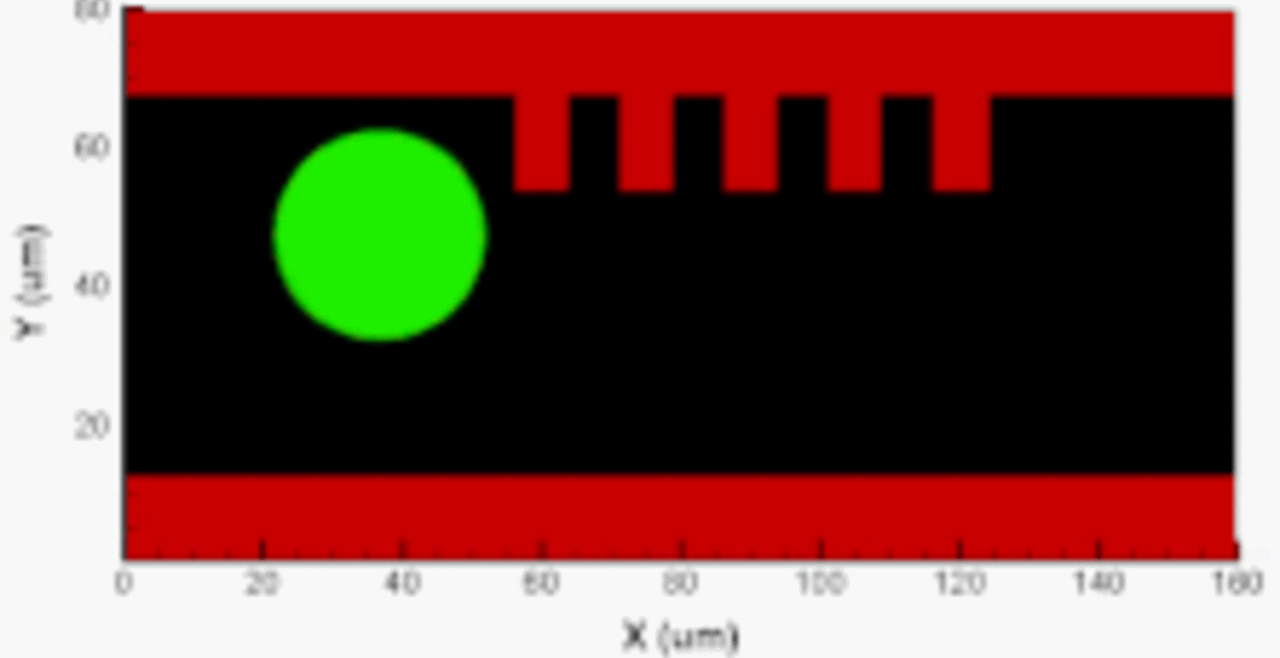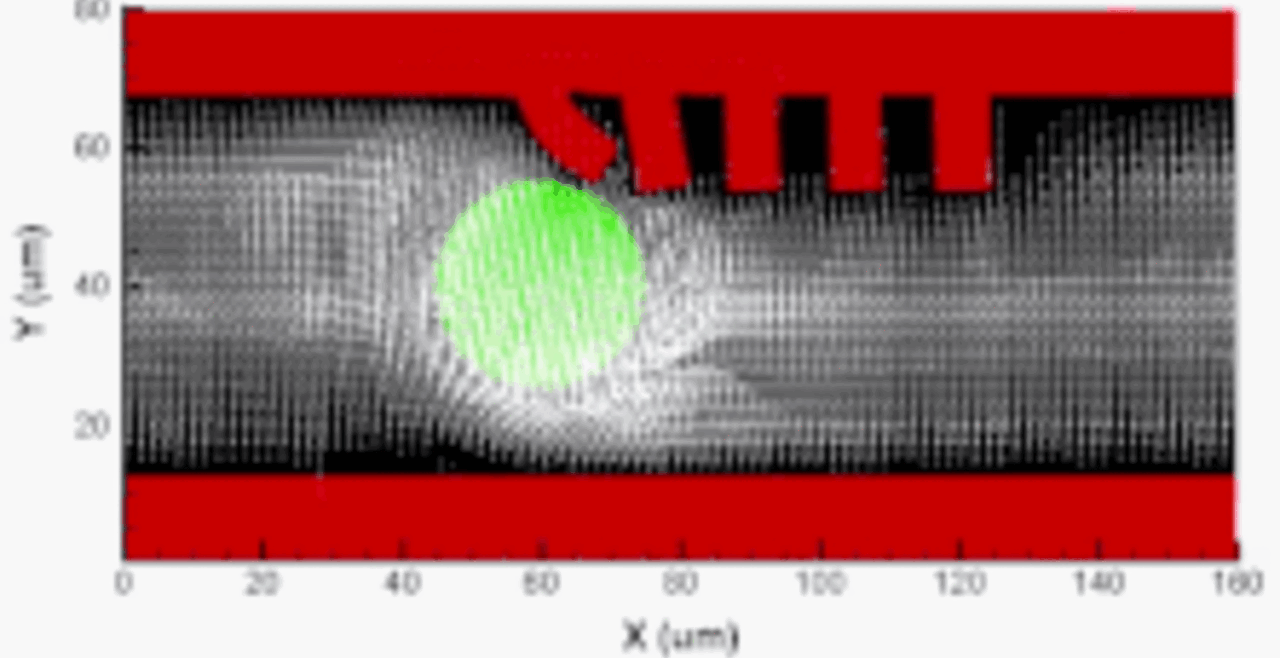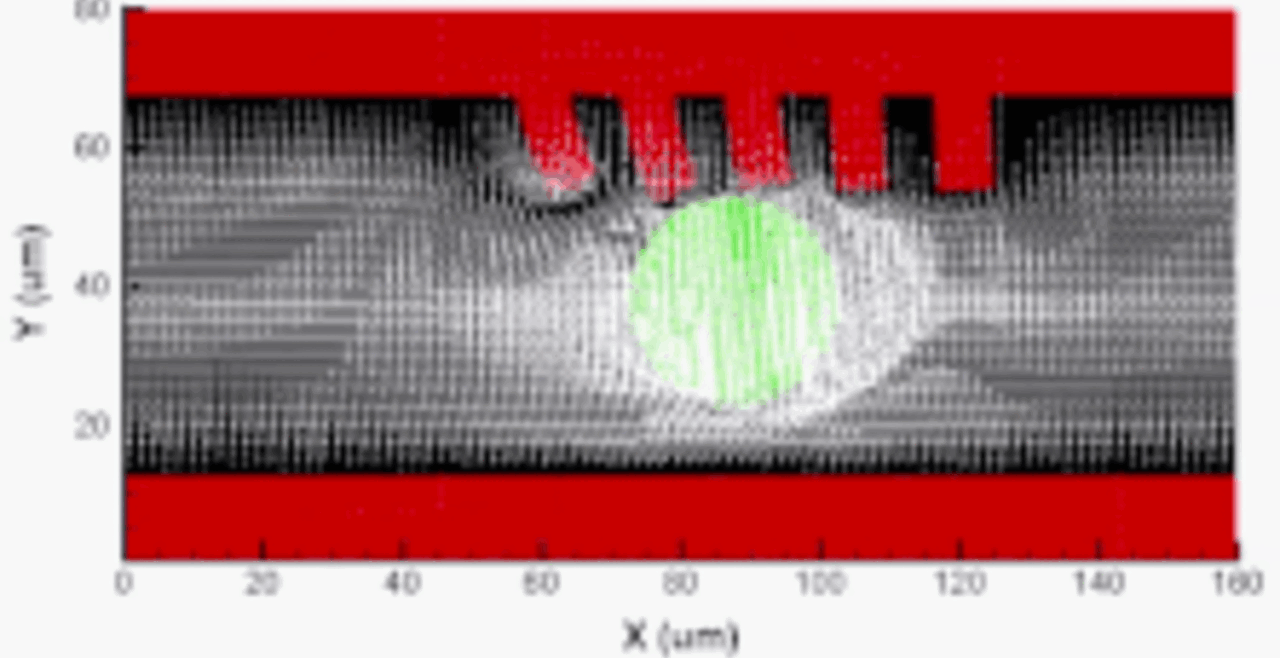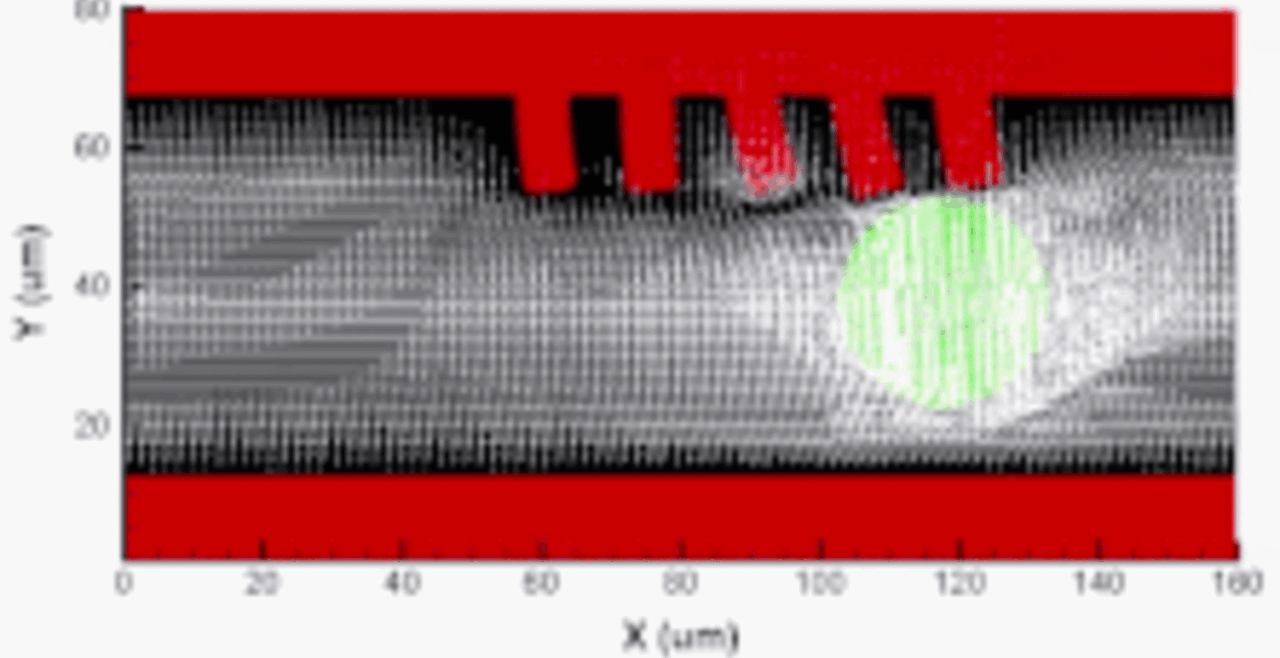
The Cellular Biomechanics and Biotransport (CBB) laboratory studies three types of human cells: 1) myeloid cells (e.g., monocytes, macrophages, mast cells), 2) vascular endothelial cells, and 3) circulating tumor cells. We are particularly interested in the morphological and phenotypic changes these cells undergo in response to various inflammatory mediators, as well as their interactions under physiological and pathophysiological flow conditions. Using endothelium-lined microfluidic systems, we study the migration, deformation, and adhesion of circulating monocytes and tumor cells, and investigate endothelial activation and dysfunction associated with atherosclerosis, inflammation, and cancer metastasis. The CBB laboratory is equipped with the Bioflux 200 microfluidic shear flow system (Fluxion Biosciences, currently Cell Microsystems), which allows up to 24 cell adhesion assays to be run in parallel. We also have experience analyzing cell migration and adhesion using microfluidic channels from other manufacturers, such as ibidi.
Our previous in vitro experiments revealed the synergistic effect of histamine and tumor necrosis factor-\(\alpha\) (TNF-\(\alpha\)) on endothelial activation and monocyte-endothelium adhesion; unique contributions of histamine to primary hemostasis; the critical role of lipopolysaccharide-activated circulating monocytes in breast cancer metastasis; and the co-activation of macrophages and mast cells by oxidized low-density lipoprotein (oxLDL). In the latter study, we demonstrated that inflammatory mediators released by oxLDL-activated myeloid cells caused significantly greater damage to vascular endothelial cells than direct exposure to oxLDL alone, highlighting the vital role of tissue-resident cells in the development of atherosclerosis. Using a combination of ELISA, adhesion, and migration assays, we recently showed that both native LDL and its oxidative form (oxLDL), especially in the presence of interleukin-6 (IL-6), can induce a pro-inflammatory, chemotactic Mox-like phenotype in human macrophages. These Mox-like macrophages promoted mast cell migration and their co-localization with macrophages, an important factor in the development of atherosclerosis and the formation of vulnerable plaques. In collaboration with Dr. Woods' laboratory (Department of Physiology, Tulane University School of Medicine), we discovered that exosomes released by diabetic smooth muscle cells promote endothelial activation and monocyte–endothelial adhesion, suggesting a potential mechanistic link between diabetes and the development of atherosclerosis.
Mox-like macrophages share a similar morphology with anti-inflammatory M2-like macrophages but exhibit a hybrid phenotype, characterized by the secretion of both pro- and anti-inflammatory factors.

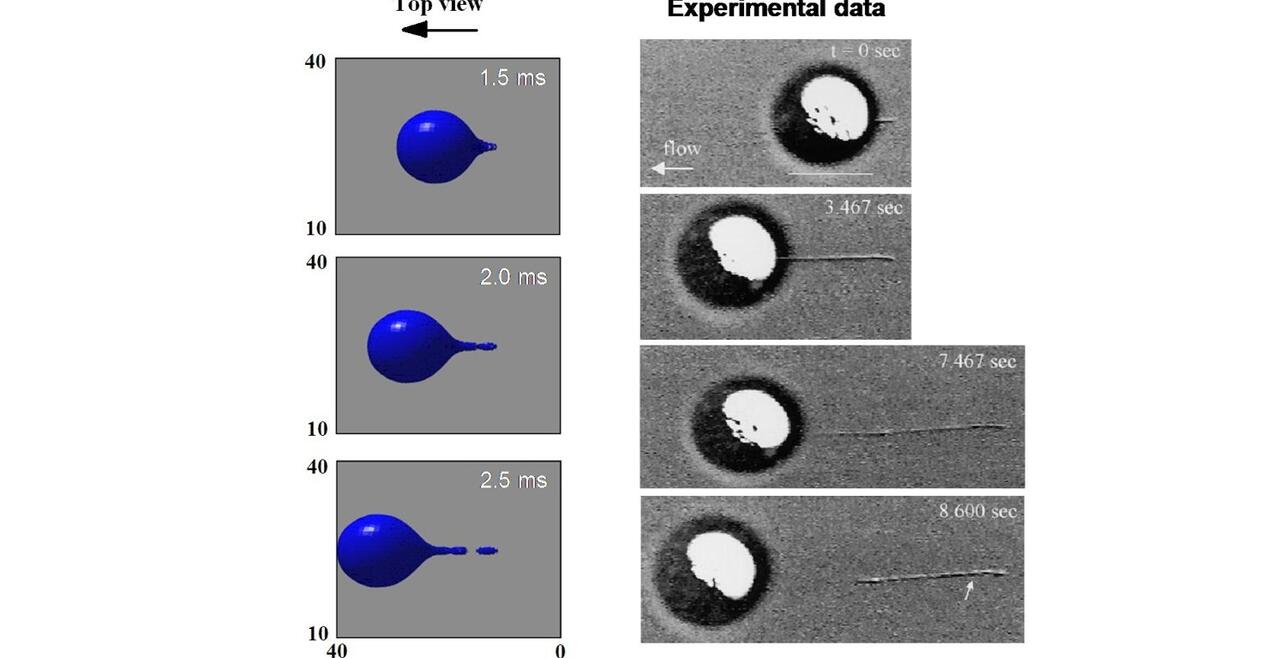
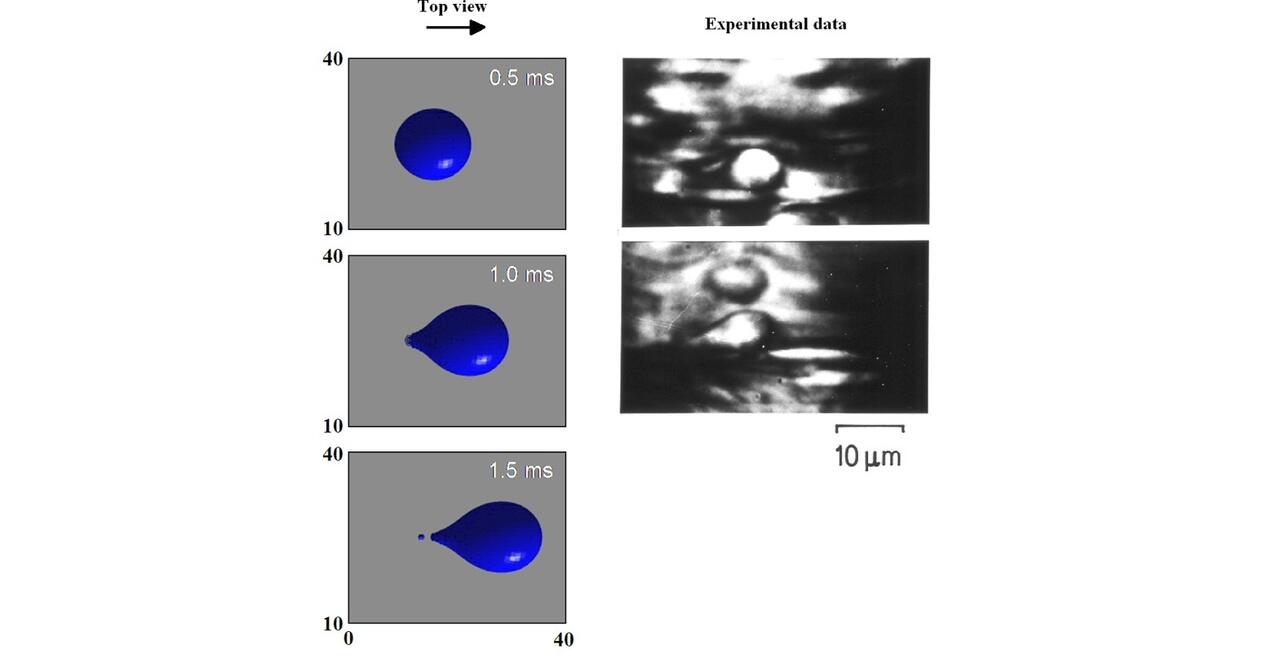
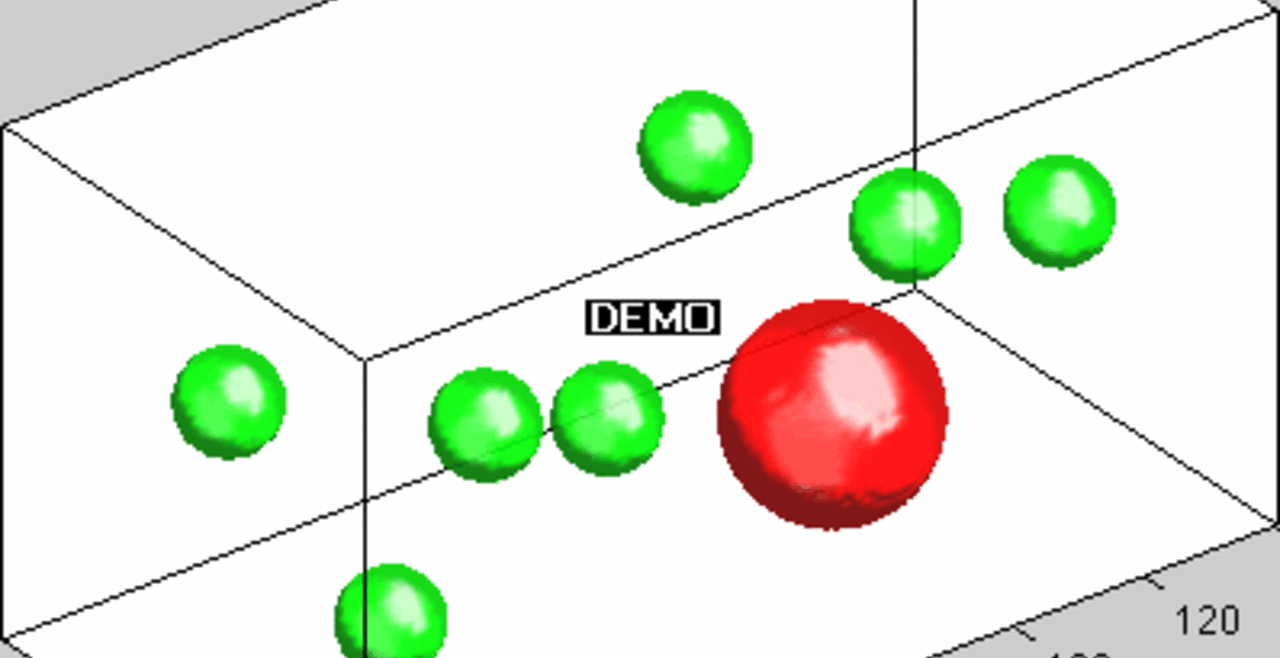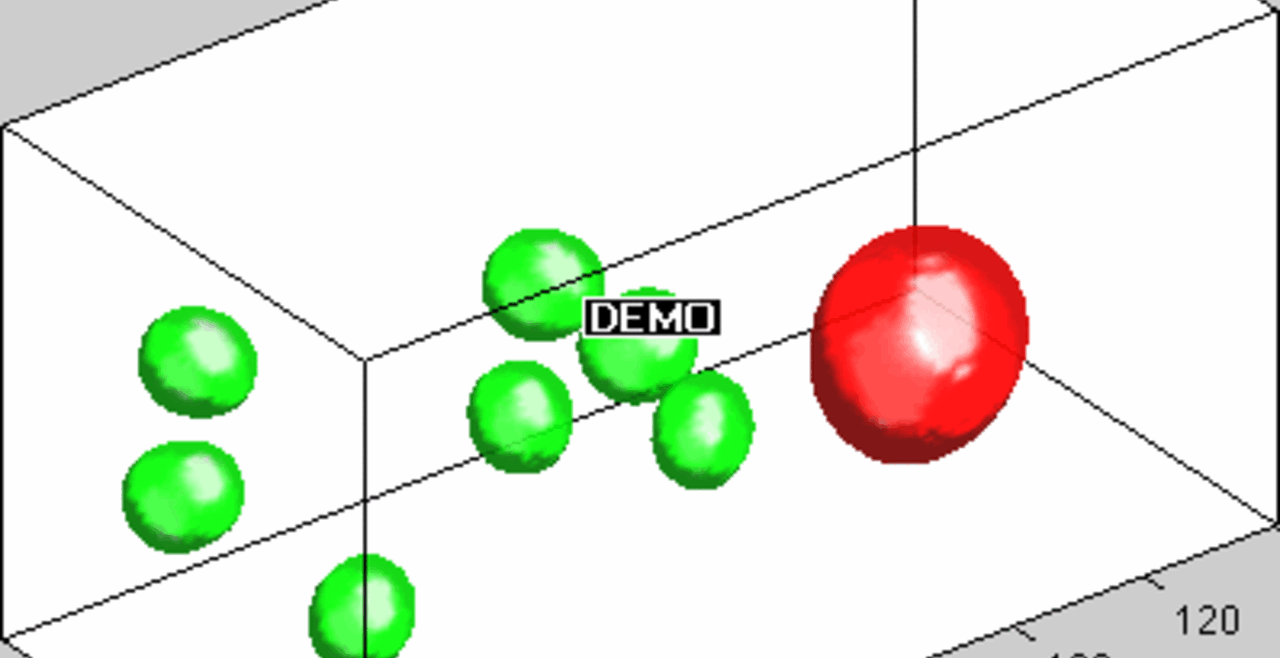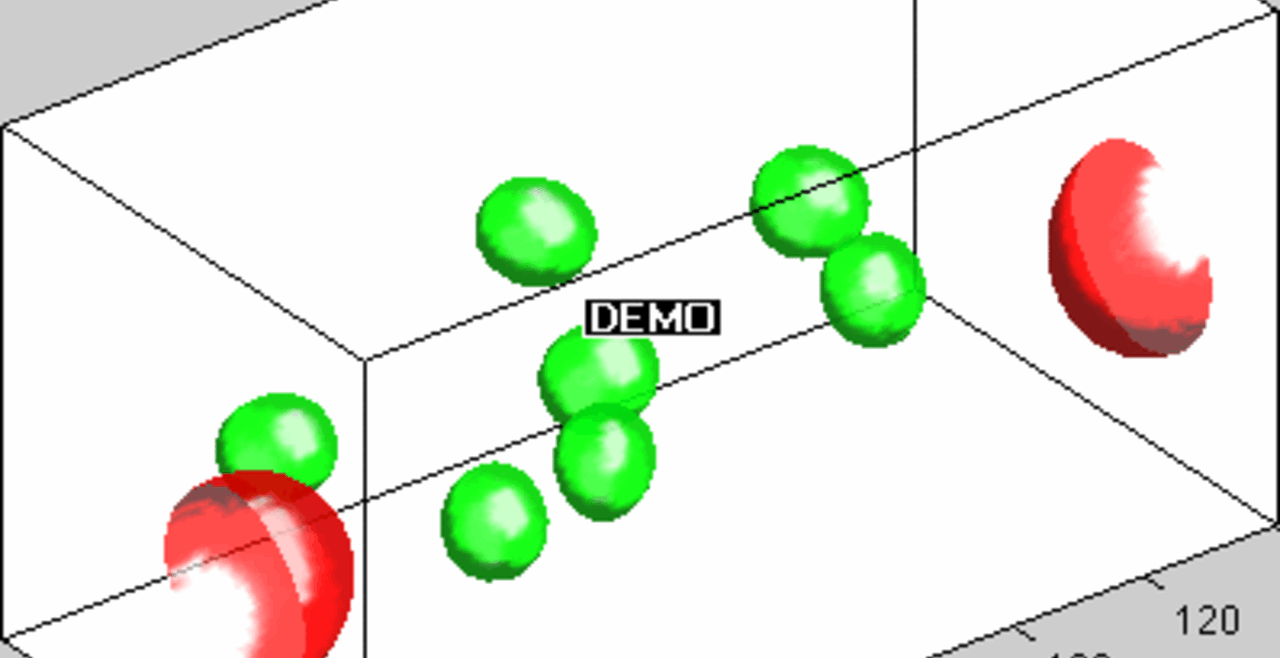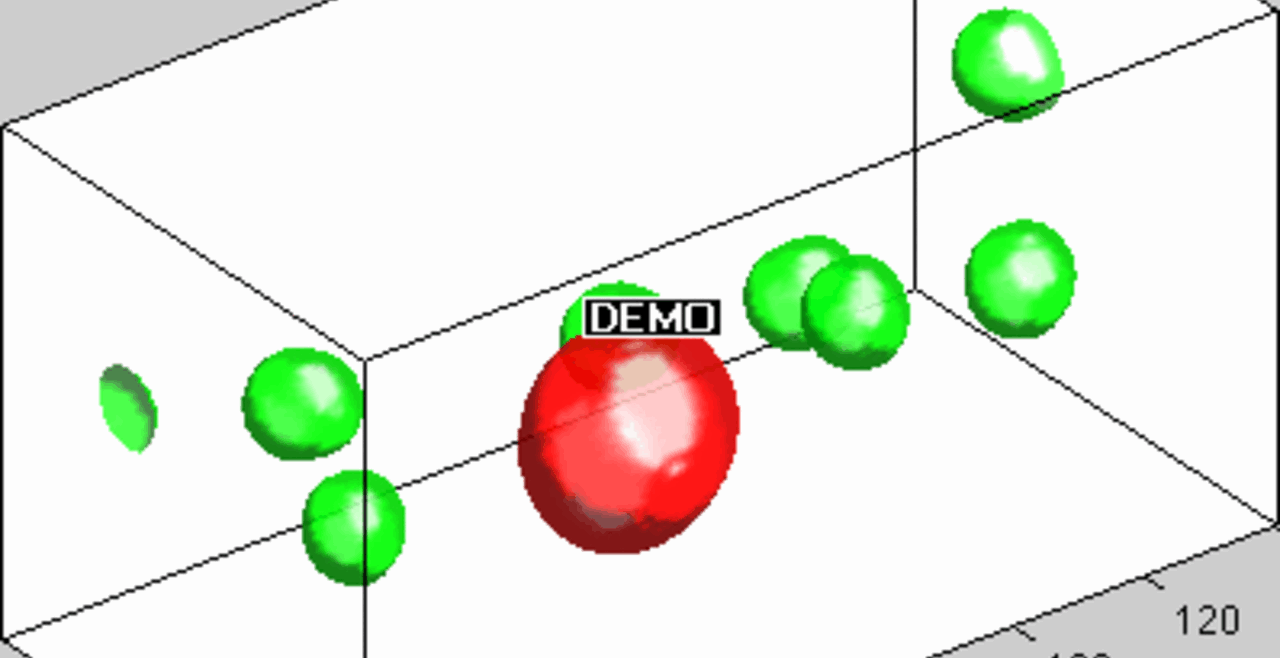
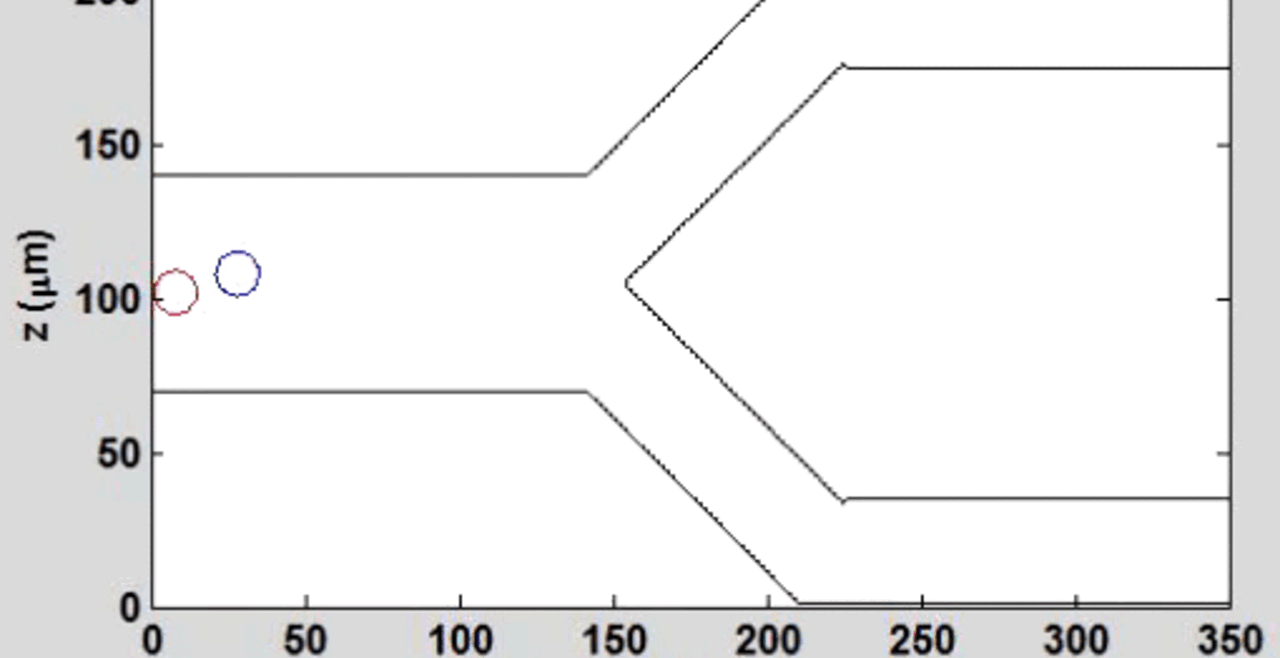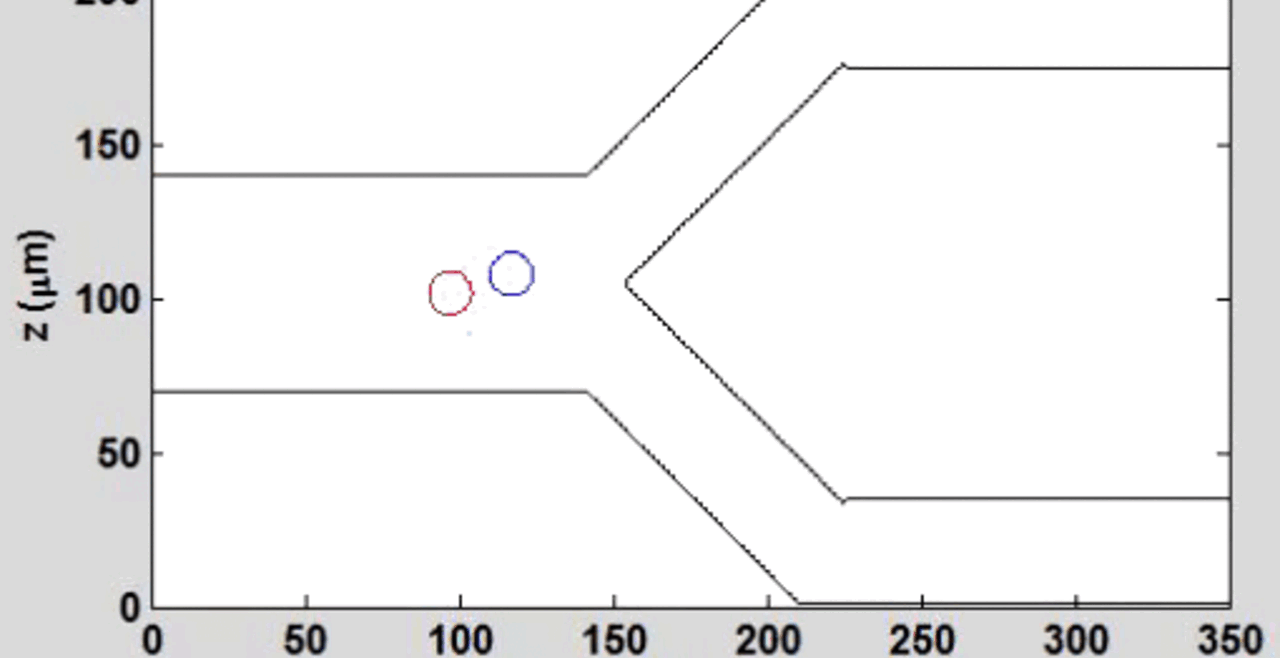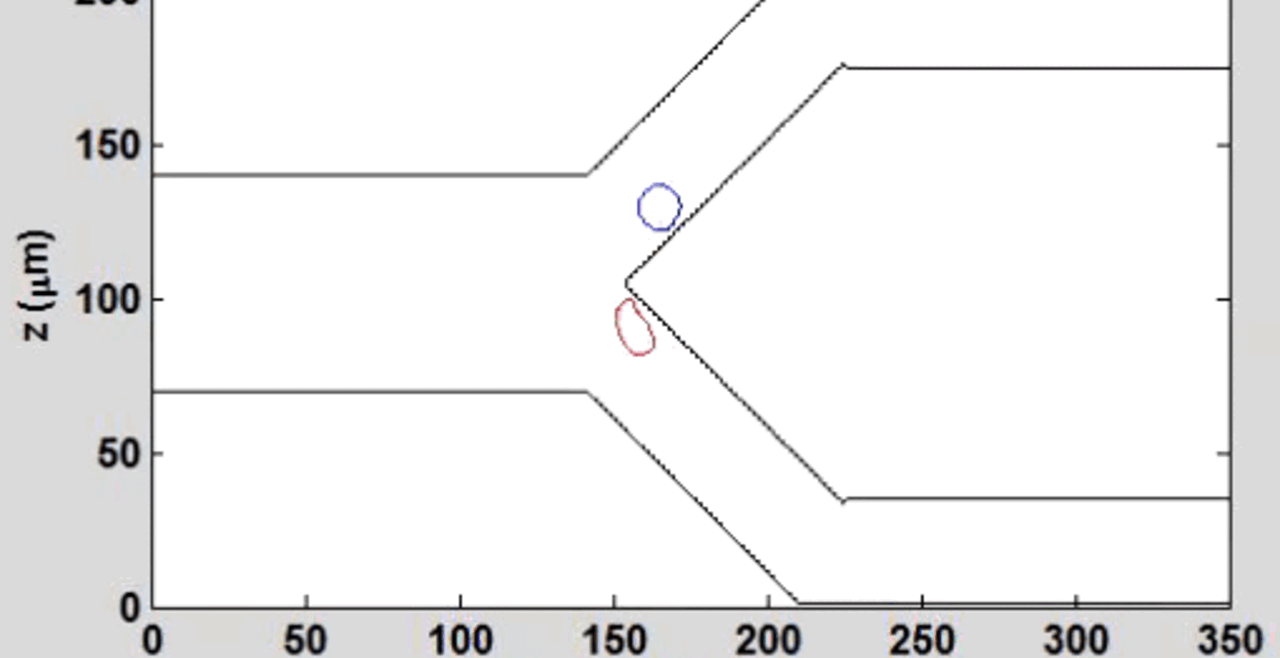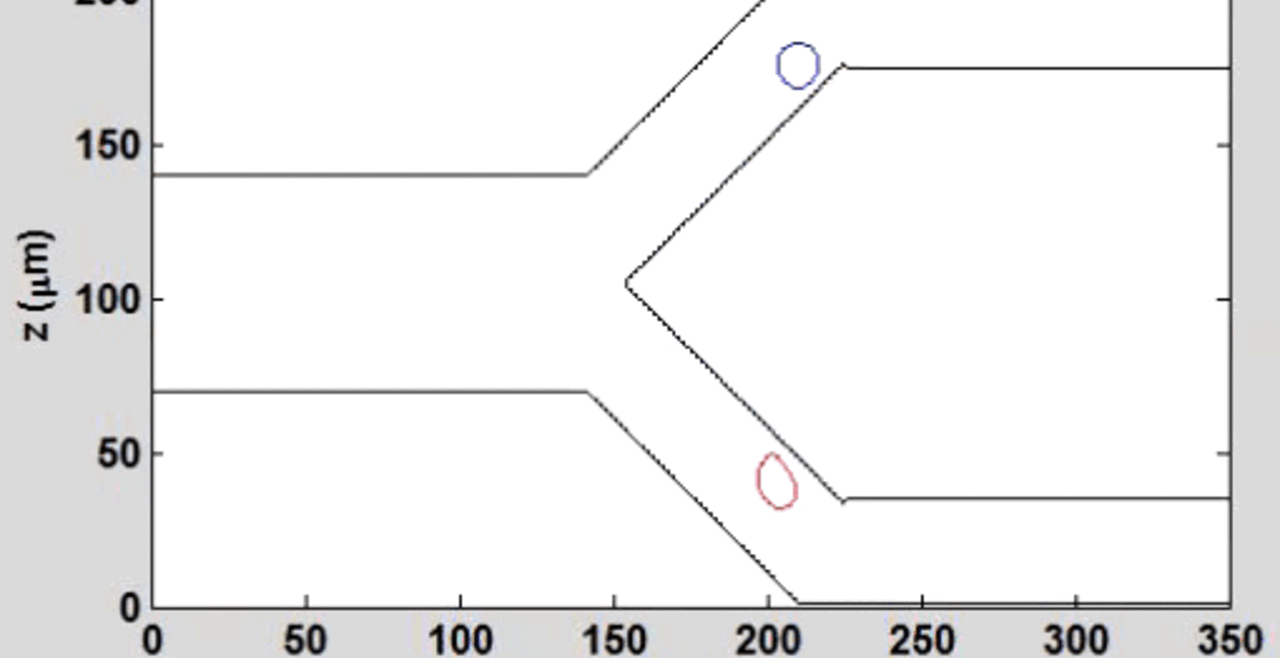
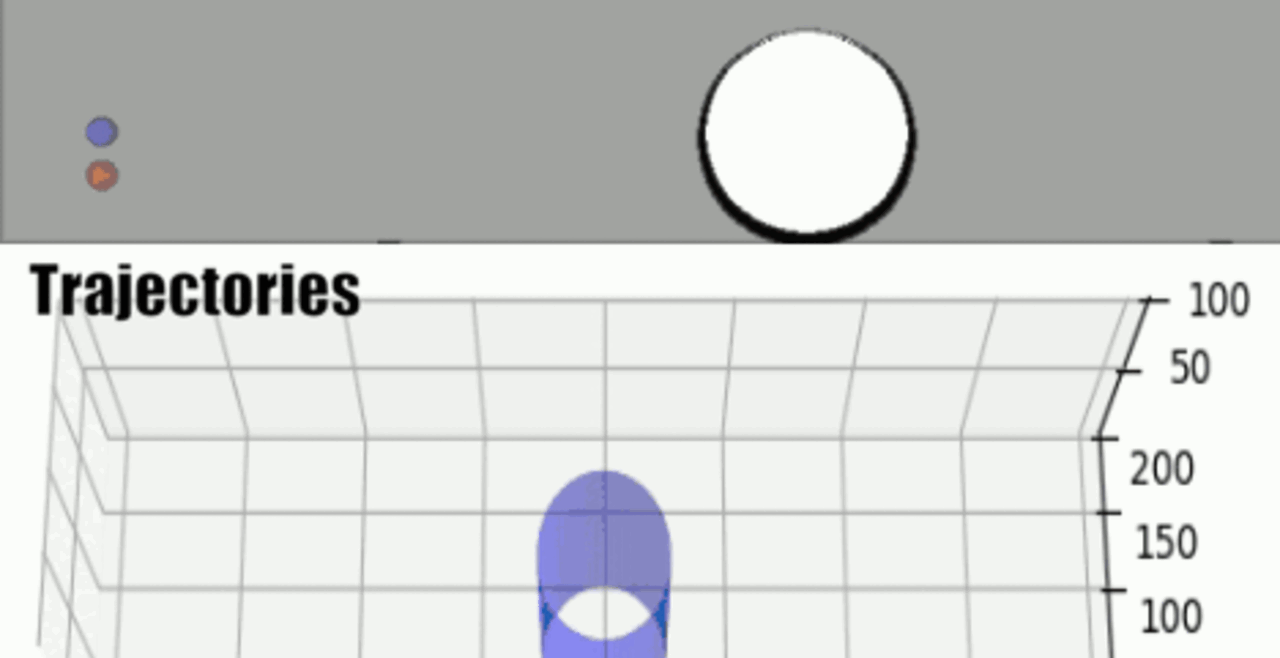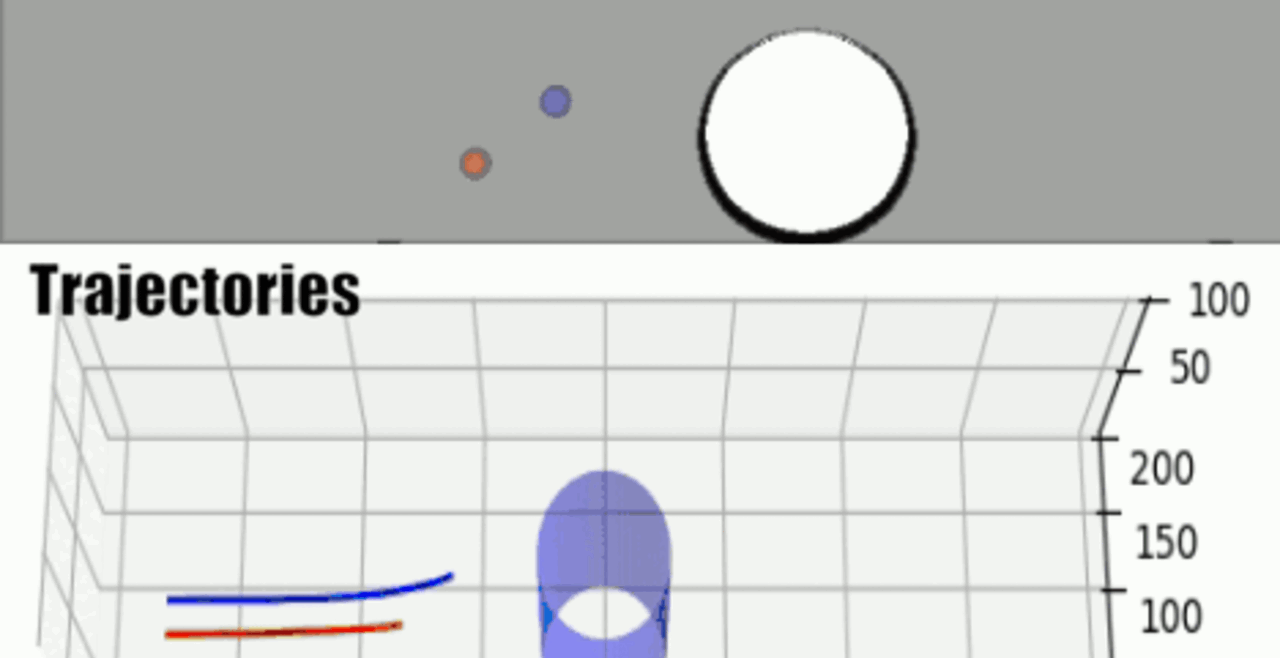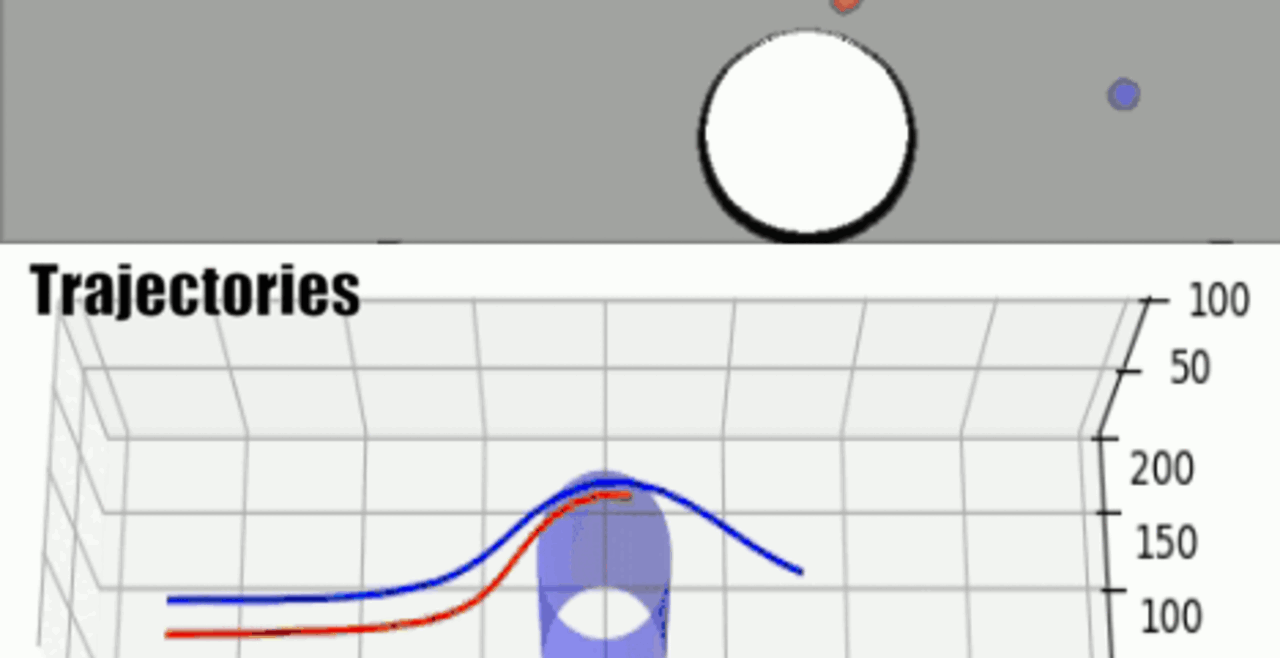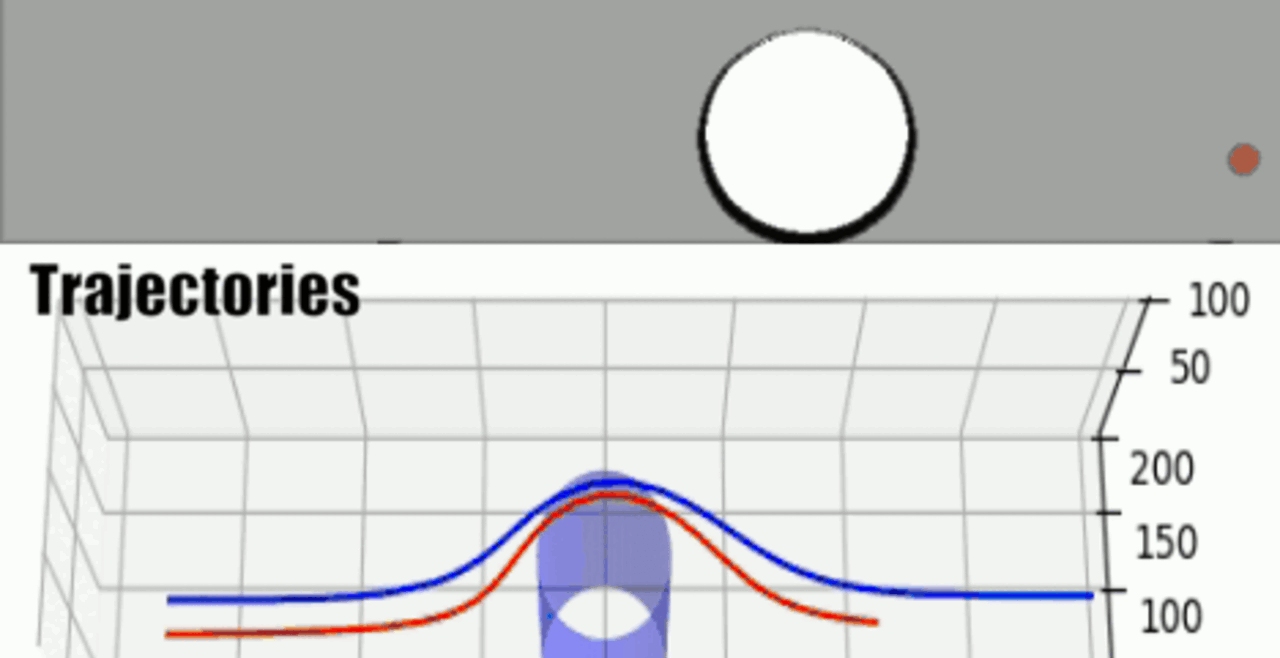
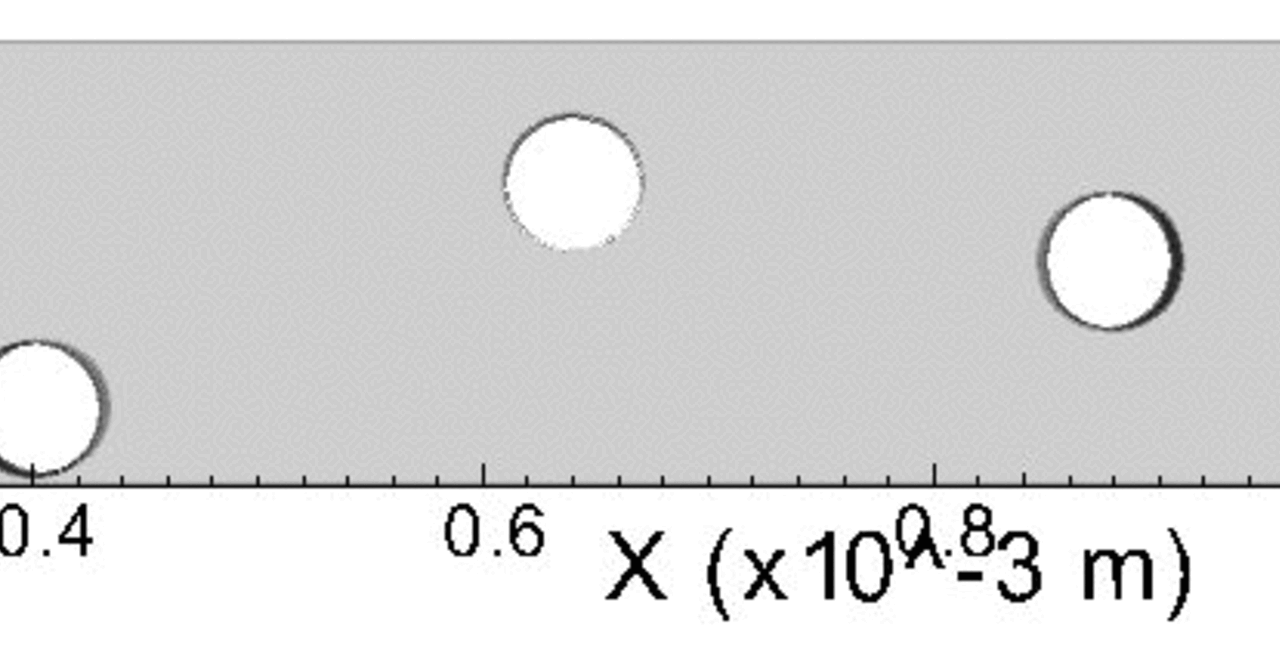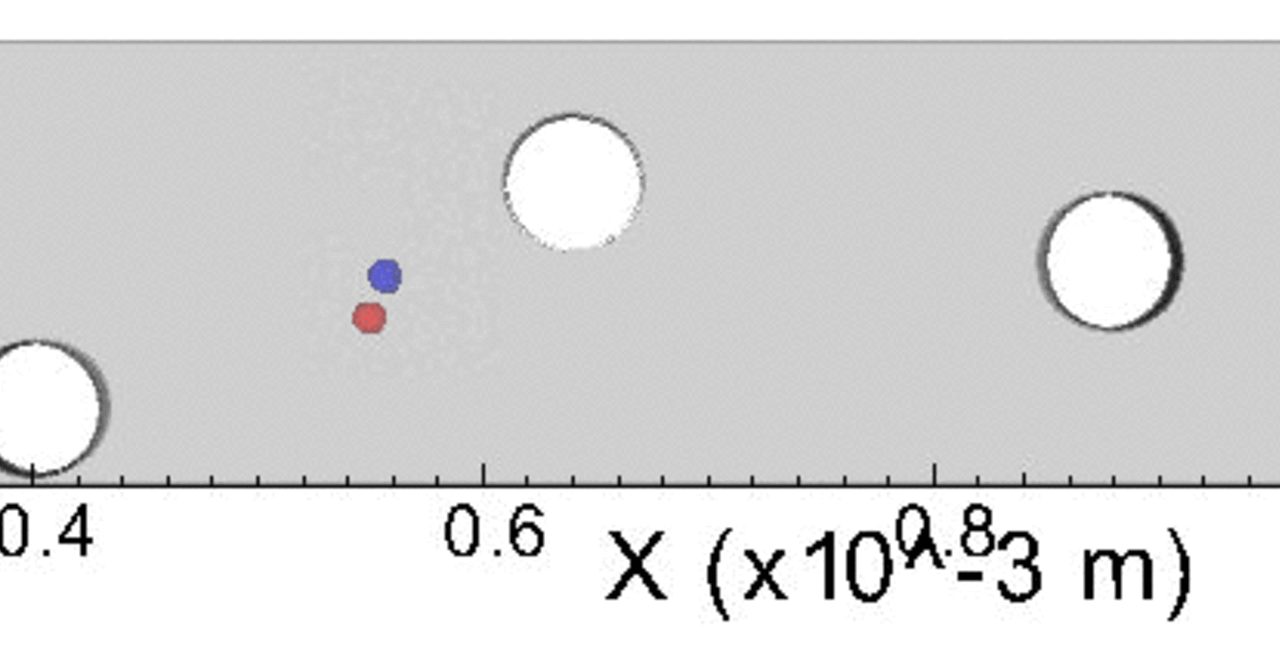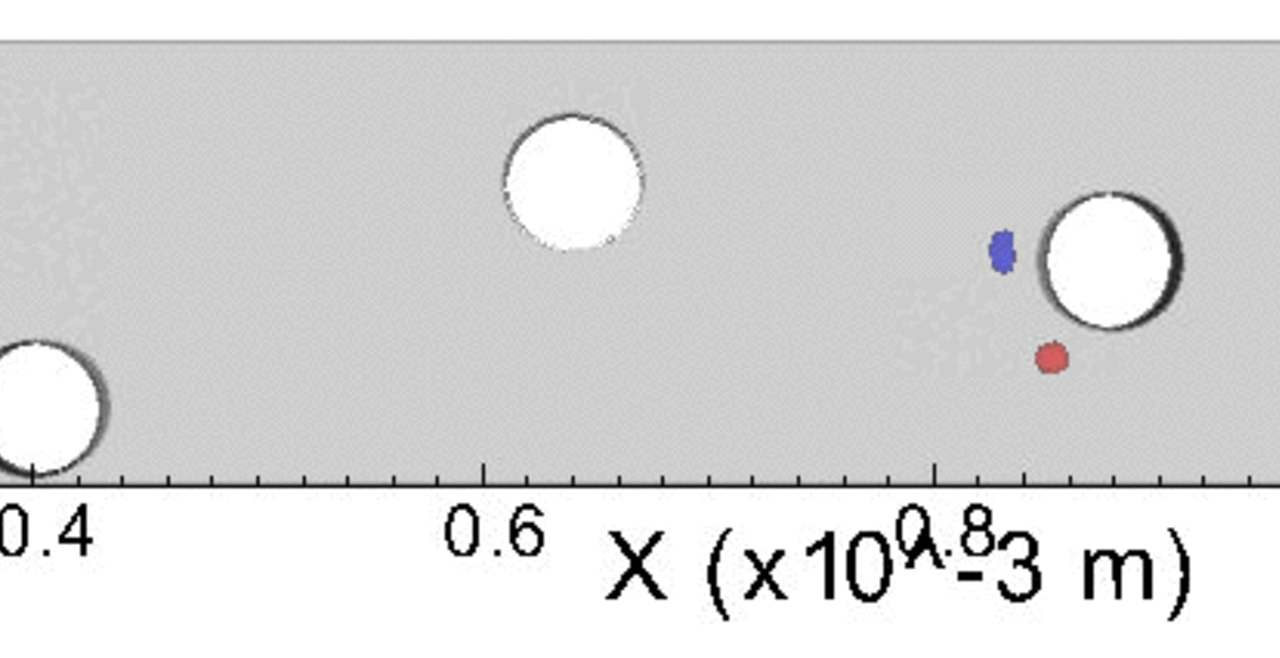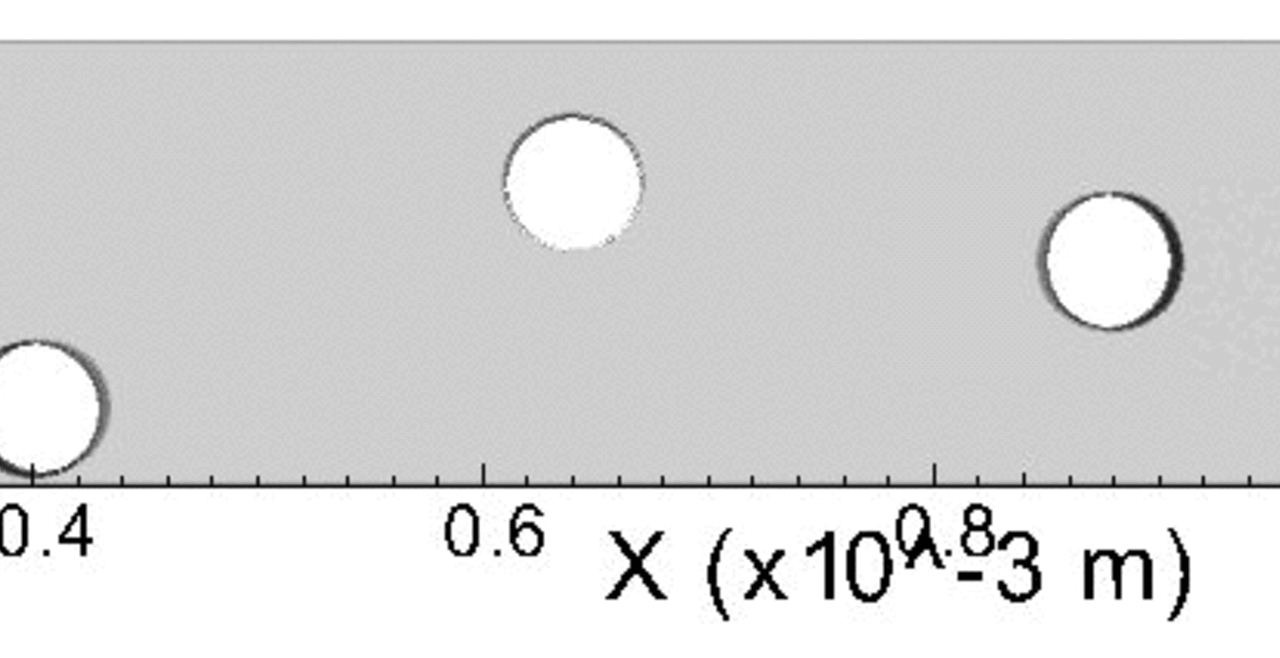
THP-1 human monocytic cells in a micro-channel lined with TNF-\(\alpha\)-activated HUVEC. Slow moving cells are rolling cells.
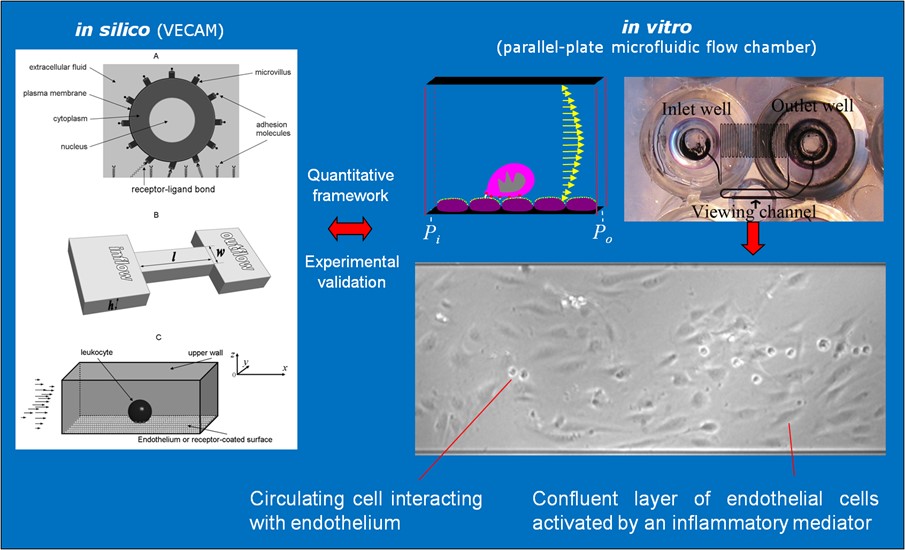
Our integrative experimental-computational approach to study vascular endothelial cells and their interactions with circulating cells.
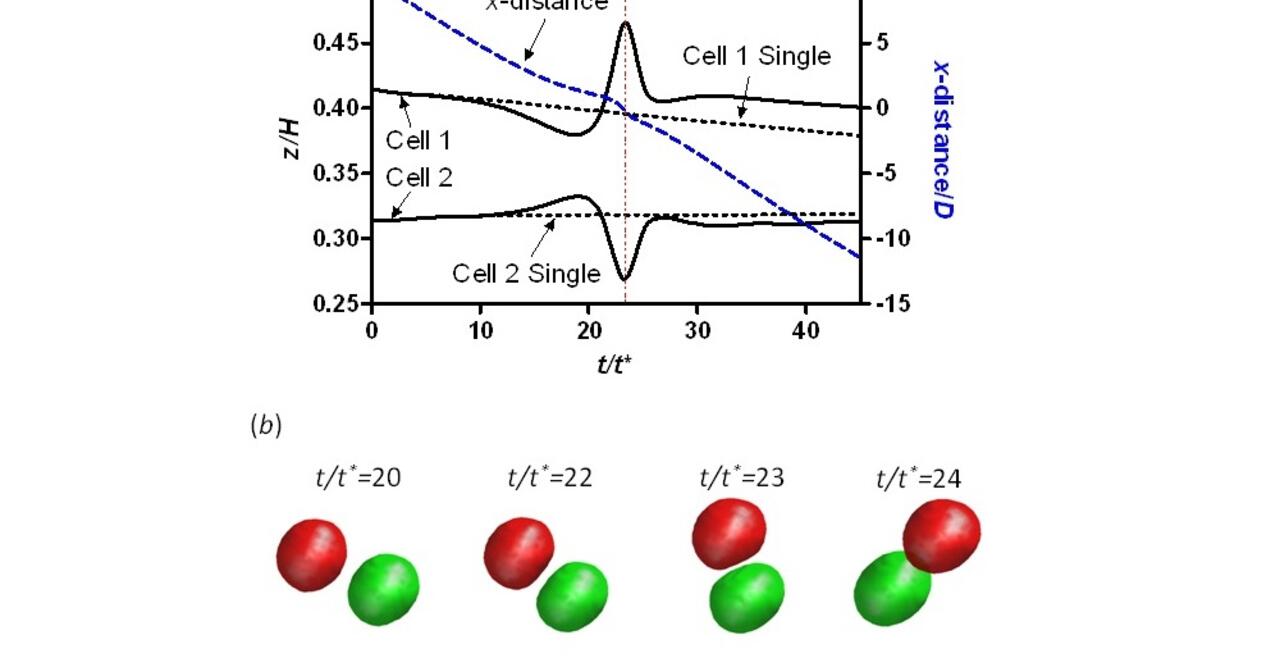
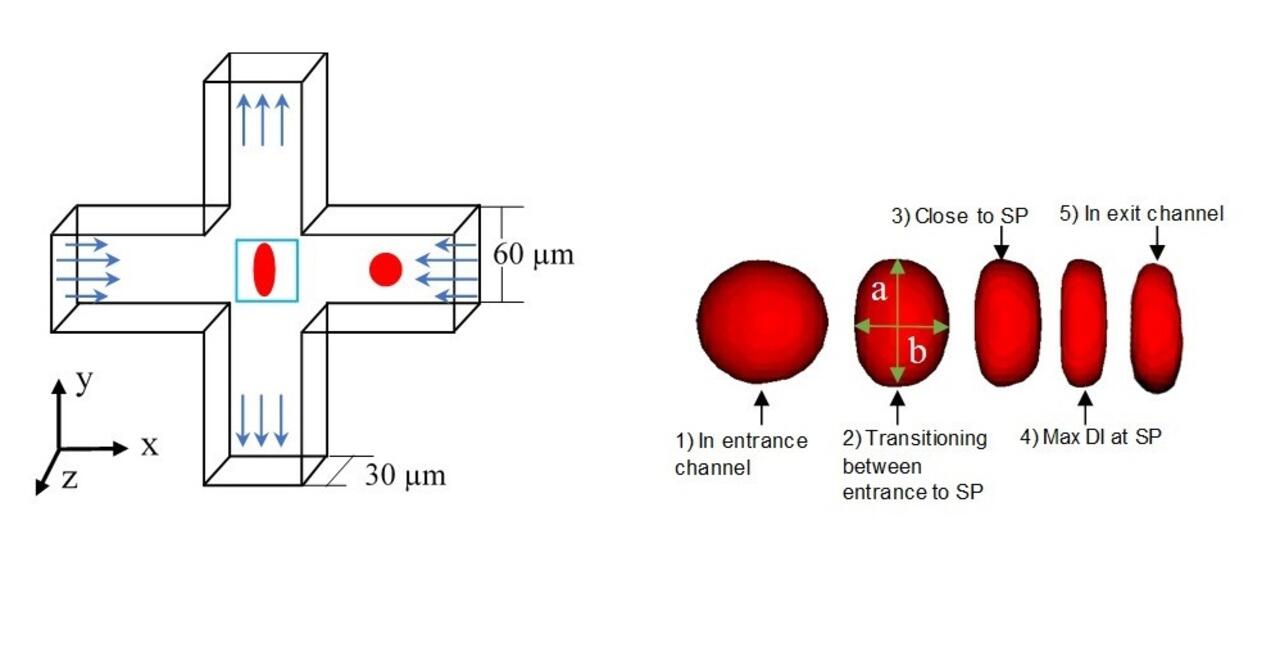
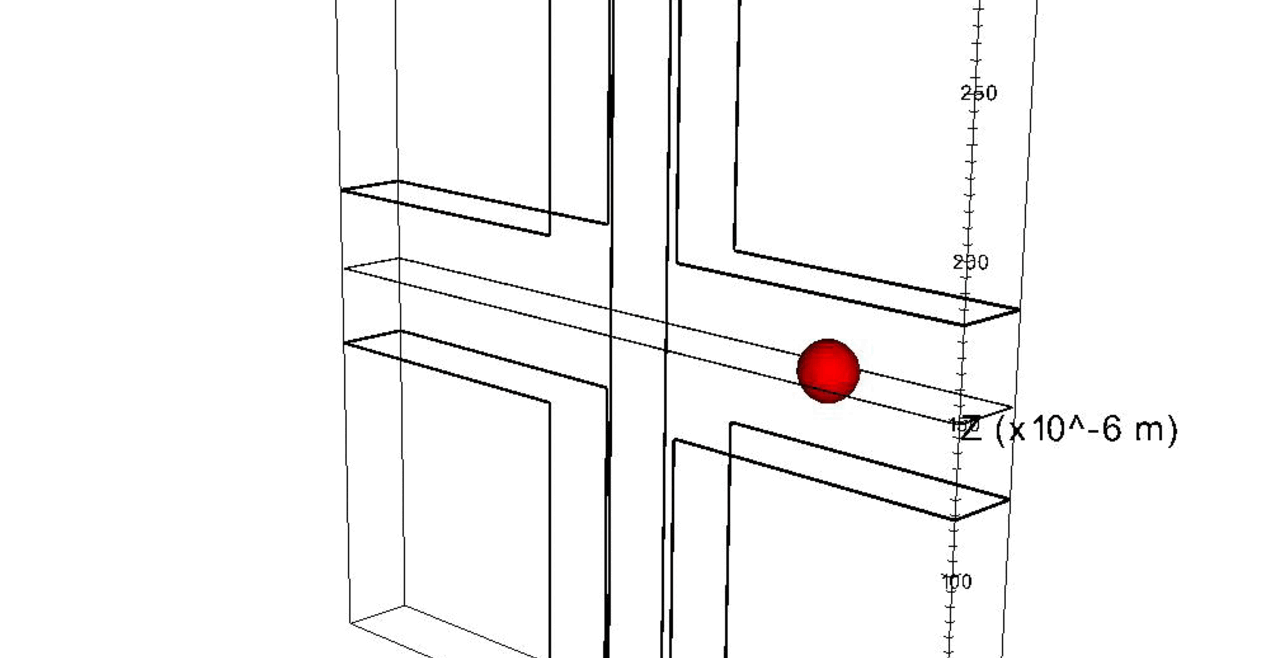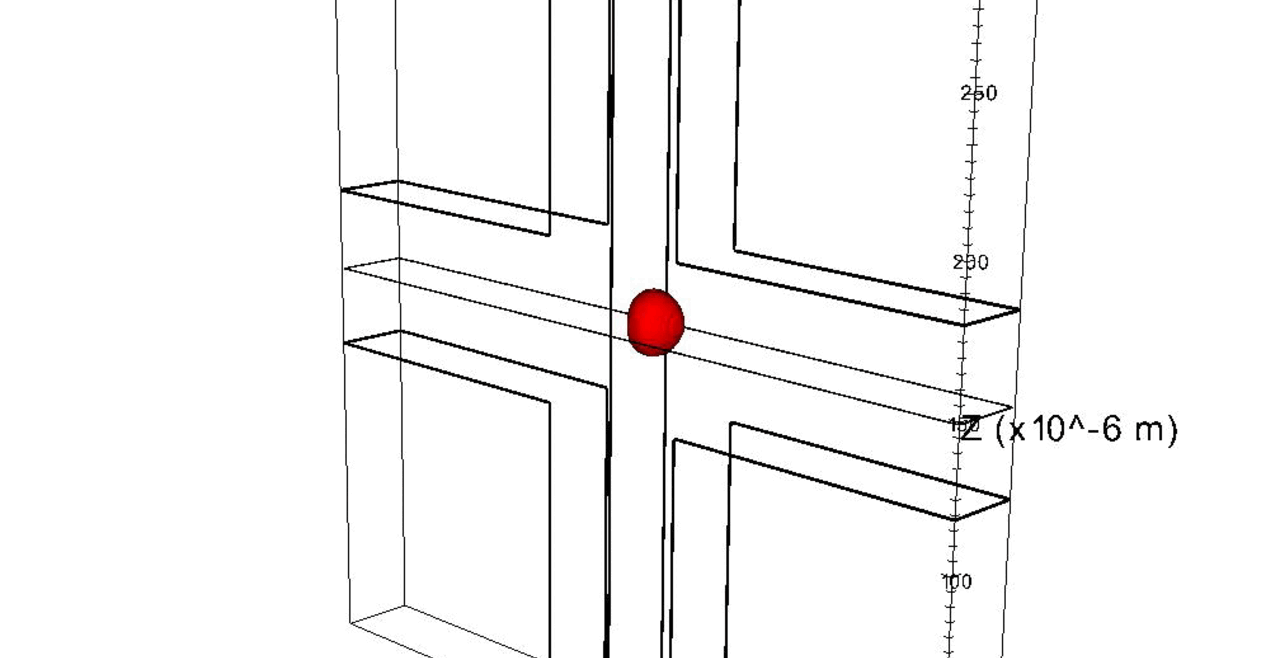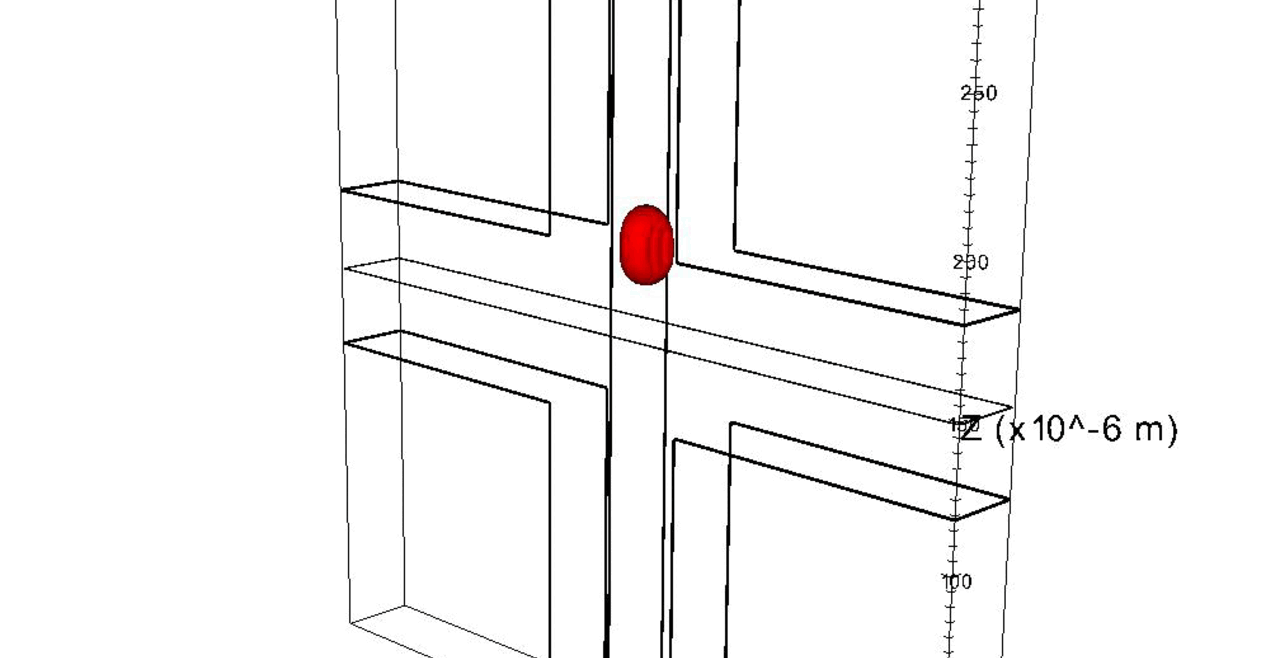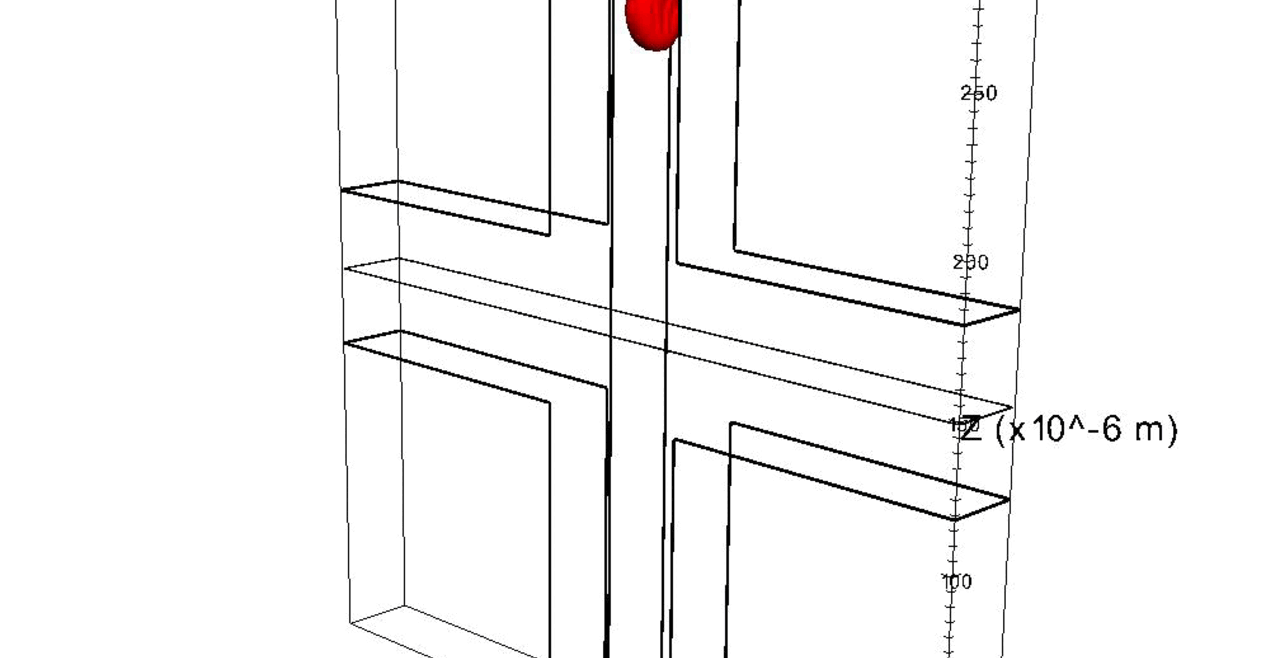
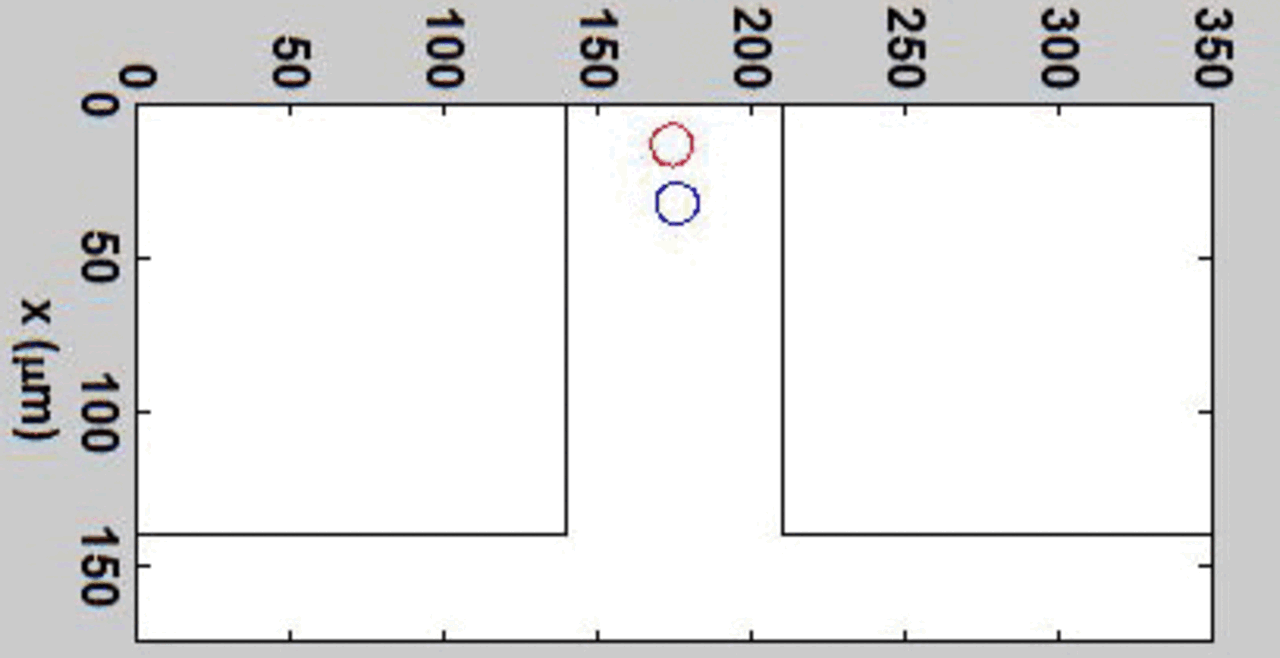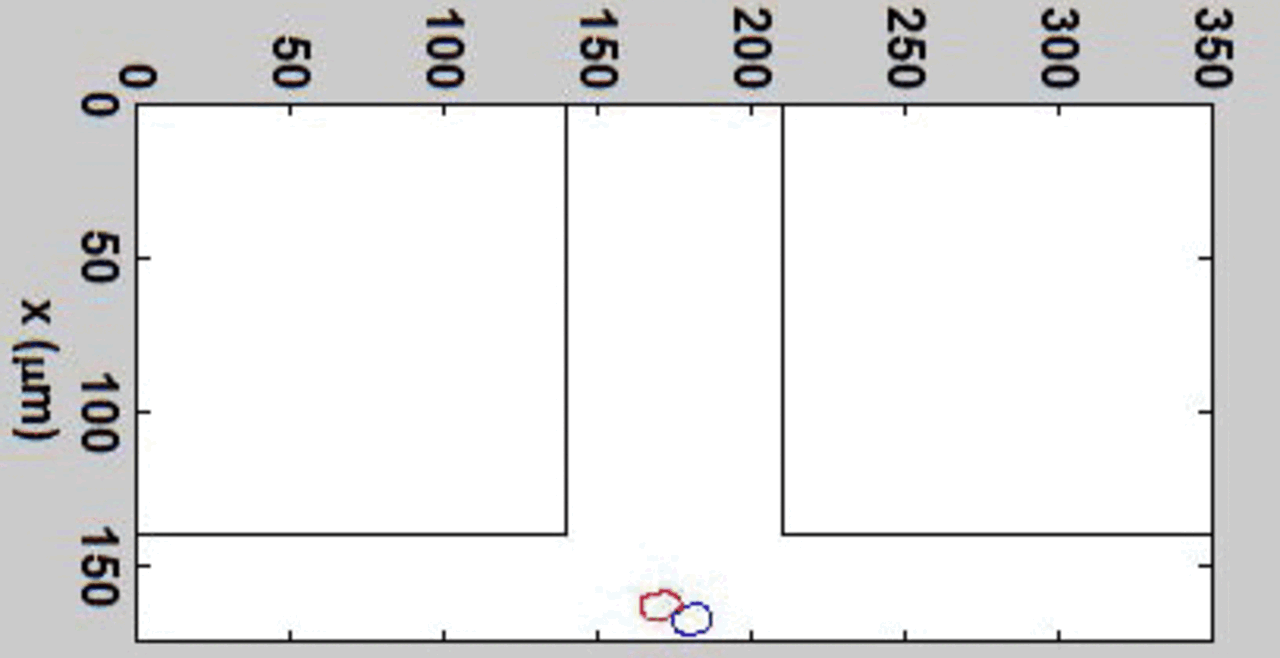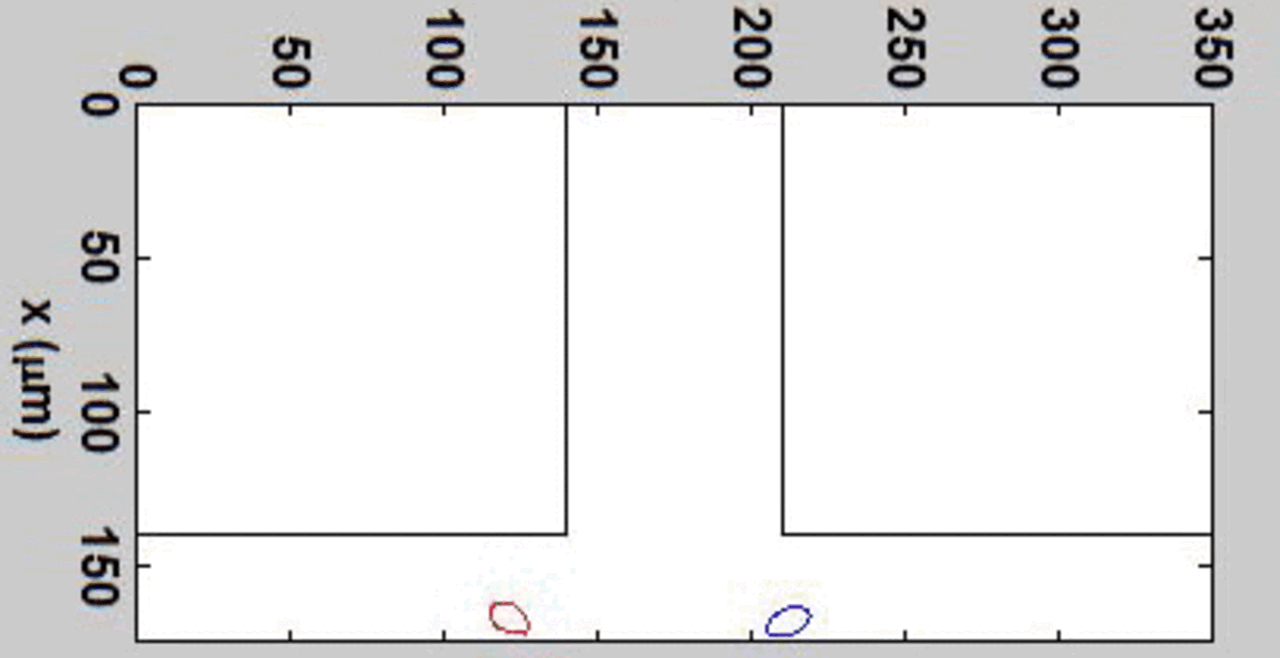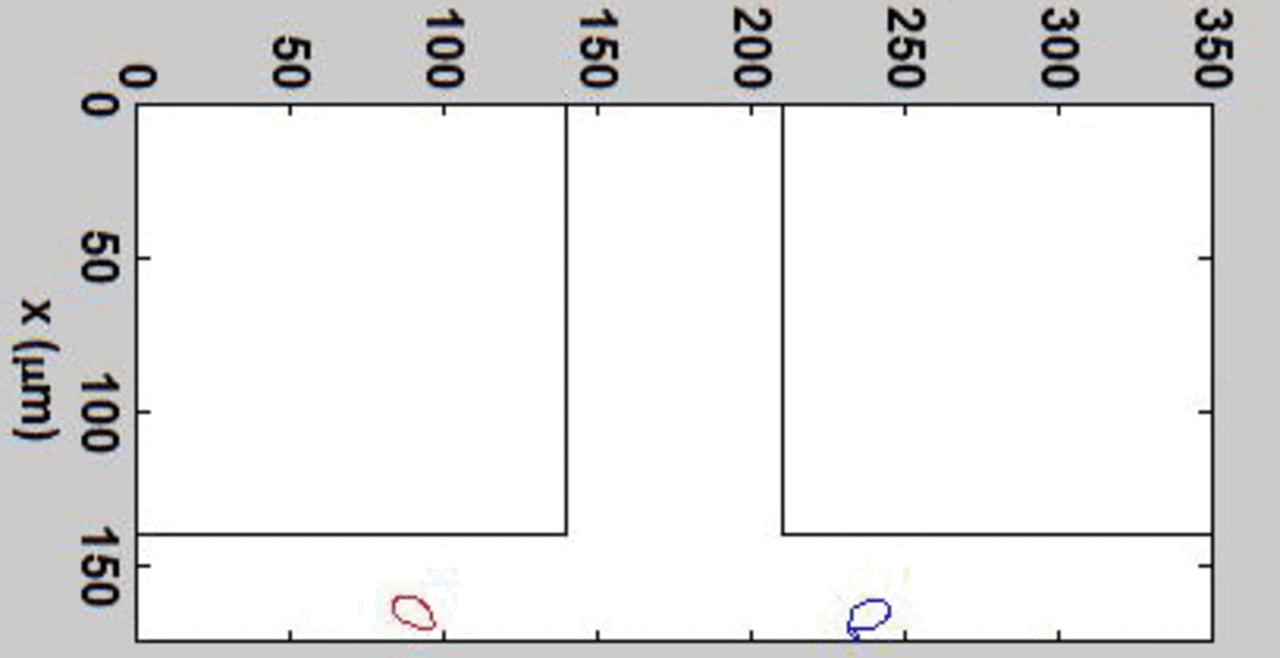
We complement our experimental work with state-of-the-art in silico modeling of cell deformation and migration. Our algorithm, the Viscoelastic Cell Adhesion Model (VECAM), realistically captures the shape changes and velocities of circulating cells as they interact with inflamed vascular endothelium. The updated version, VECAM-Active, can simulate both passive and active deformation of adherent cells, chemotactic movement, adhesion to compliant substrates, and the formation of finger-like cell projections, closely matching observations from micropipette experiments on chemoattractant-induced leukocyte motion. It also accommodates multiple circulating cells with varying deformability and size. Over the past decade, VECAM has been applied to problems such as cell separation and sorting in bifurcating micro-channels and micro-channels with sequenced pillars, as well as cell motion and deformation in microfluidic and quantitative deformability cytometry techniques. With the incorporation of cell spreading and contractility models, VECAM-Active now supports applications in haptotactic migration and traction force microscopy.
Research Area 2: Therapeutic Ultrasound
One of the key focus areas of the Biomedical Acoustics (BA) laboratory is therapeutic ultrasound, i.e., the use of high-frequency acoustic waves at intensities exceeding diagnostic levels for therapeutic applications. The laboratory is equipped with both focused and unfocused ultrasound systems, along with peripheral instrumentation for characterizing high-intensity acoustic fields. We have extensive experience in testing ultrasound bioeffects in cultured cells, ex vivo tissues, and in vivo models. Our previous work includes studies on enhanced drug delivery using focused ultrasound (FUS), tumor ablation and other anti-cancer effects of high-intensity FUS, and nerve regeneration by low- to mid-intensity ultrasound. Our data show that therapeutic ultrasound induces phenotypic changes in living cells by mechanically disrupting their intracellular structures. Recently, we discovered that FUS-induced mechanical disruption leads to chromatin remodeling, which sensitizes human breast and prostate cancer cells to hormone therapy. Additionally, our results demonstrate that combining FUS with chemical therapeutic agents (e.g., ethanol, NF-κB inhibitors) significantly reduces the aggressiveness of human liver and prostate cancer cells. We refer to this combination approach as mechanochemical disruption (MCD). Overall, our findings suggest that FUS has the potential to induce reprogramming of somatic cells.
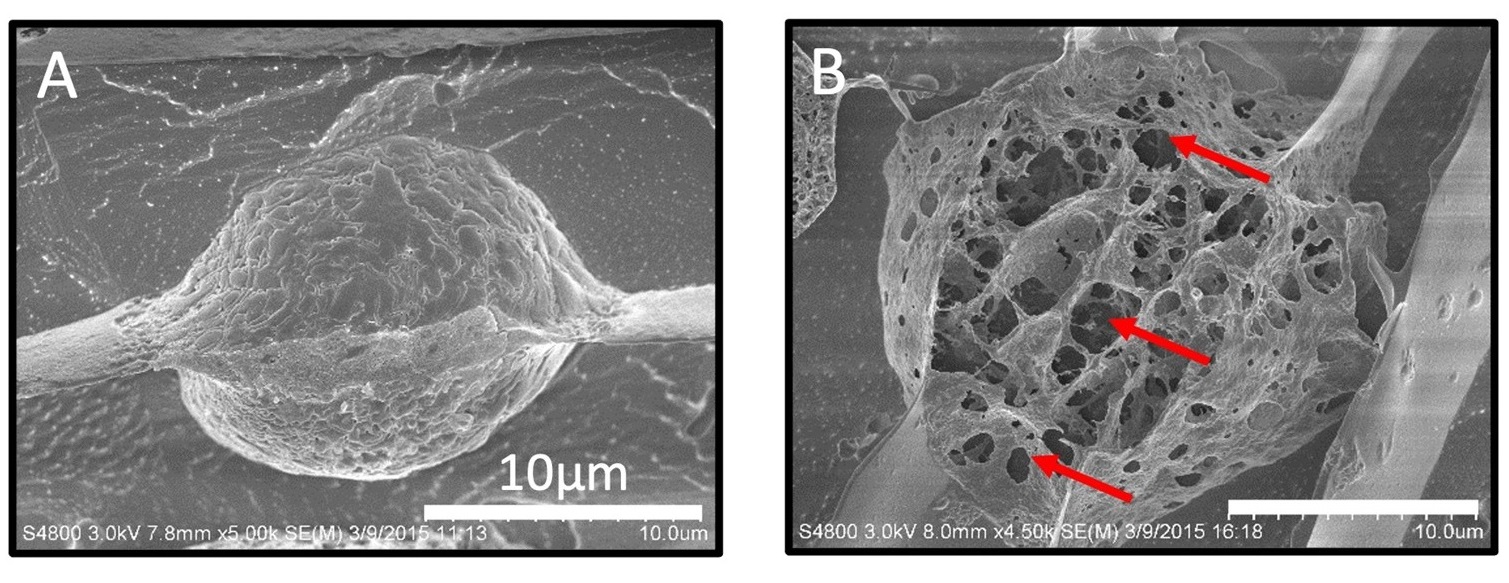
Mechanical disruption of the plasma membrane and cytoskeleton of human prostate cancer cells by FUS. Shown are cryogenic scanning electron microscopy (cryo-SEM) images of DU-145 cells either untreated (A) or treated by FUS at a frequency of 1.1 MHz and acoustic intensity of 0.7 kW/cm2 (B). From Arora et al. Mol. Pharmaceutics 13, 3080-3090 (2016).
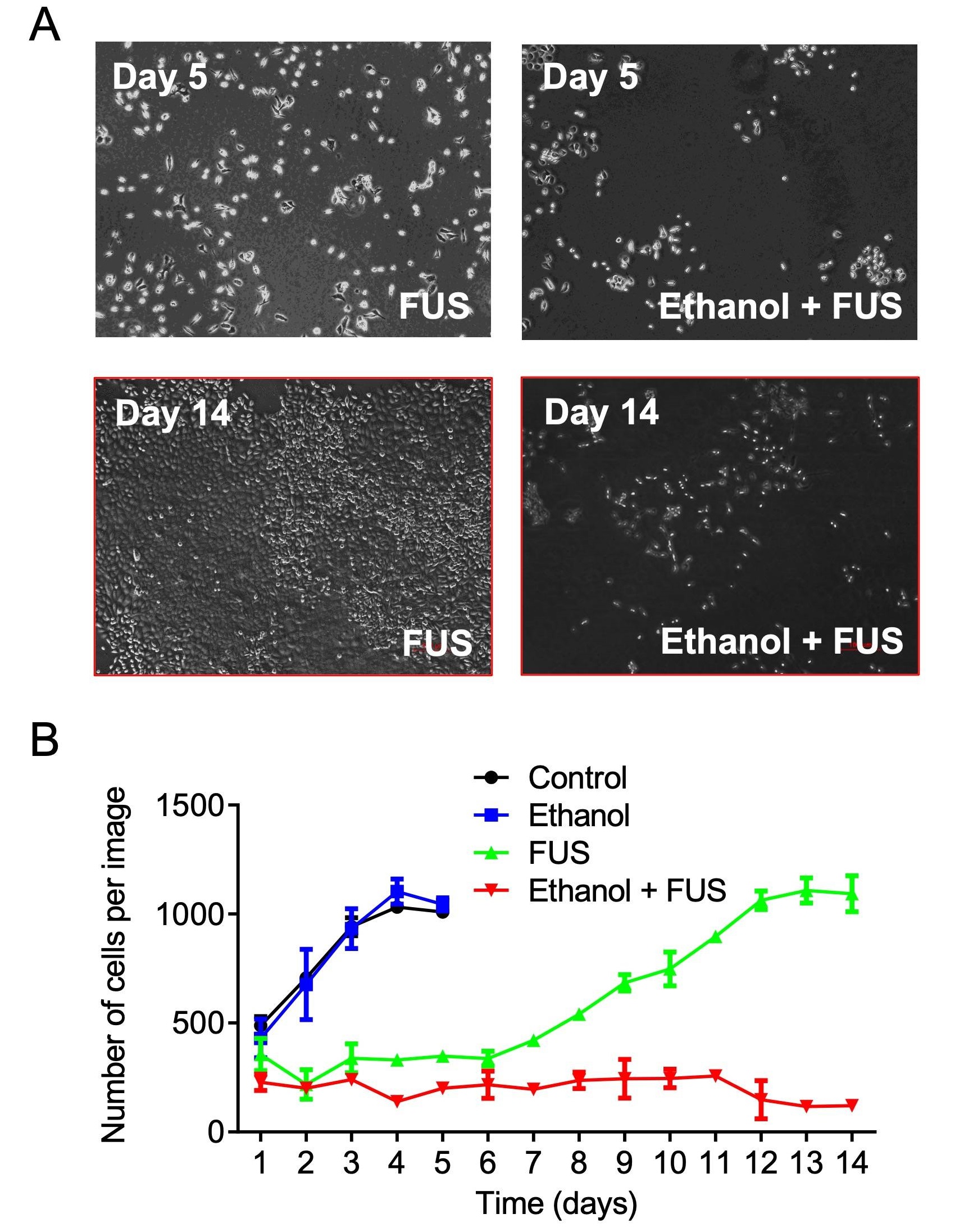
Proliferation of DU-145 human prostate cancer cells after exposure to ethanol, FUS, or their combination (MCD). Cells treated with FUS alone recover and resume growth within one week post-treatment, whereas the proliferation remains suppressed in MCD-treated cells even after two weeks. From Murad et al. Mol. Cancer Res. 17, 1087-1101 (2019).
Using DU-145 prostate cancer cells, we demonstrated that FUS significantly enhanced the cellular uptake of temperature-sensitive liposomes (TSLs) loaded with Sorafenib. Beyond improved uptake, FUS exhibited a strong synergistic effect with TSLs in killing cancer cells. When combined with Sorafenib-loaded TSLs, FUS treatment reduced DU-145 cell viability to below 10%, markedly lower than the viability observed with free Sorafenib, Sorafenib-loaded TSLs alone, or FUS alone. This synergy was attributed to FUS-induced mechanical disruption of the cells. Similar effects were observed in renal cancer cells.
When studying a combination of ethanol and FUS (MCD treatment), we observed a dramatic regression of 1-cm-diameter prostate and liver tumors in a xenograft mouse model to no-caliper-measurable size within 14 days of this treatment, while tumors continued to grow in the sham, ethanol alone, and FUS alone groups. We found that prostate cancer cells (DU-145, PC-3, C4-2B) exposed to the MCD treatment overexpressed death receptors TNFR1 and FasR and experienced a significant decrease in 1) viability and proliferation rate; 2) expression of adhesion molecules and metastatic markers (CD29, CD44); 3) tumorigenic ability; 4) capacity to adhere to vascular endothelium; and 5) ability to migrate, as compared to cells exposed to individual treatments. Similar results were obtained for liver cancer cells Hep3B, PLC/PRF/5).
Our recent experiments demonstrate that focused ultrasound (FUS) can sensitize resistant human breast and prostate cancer cells to hormone therapy. In Tamoxifen-resistant breast cancer cells, for example, FUS activates ESR1, the gene encoding estrogen receptor α, suggesting that FUS disrupts heterochromatin, a compact, transcriptionally repressive form of chromatin. This finding indicates that FUS may facilitate somatic cell reprogramming by promoting a shift toward the open, transcriptionally active euchromatin state. Our current research focuses on this “mechanochemical reprogramming” approach, which combines FUS with chemical agents to induce and stabilize this chromatin remodeling.
Additionally, we have begun investigating the effects of low- to mid-intensity ultrasound on neurons in the central nervous system, with the goal of developing an MCD-like therapy to aid recovery in patients with spinal cord injuries. Our in vitro studies using rat cortical neurons and rat neuroendocrine (PC-12) cells differentiated into neurons demonstrate that ultrasound can increase neurite density and length, as well as enhance the number of connections between neurons.
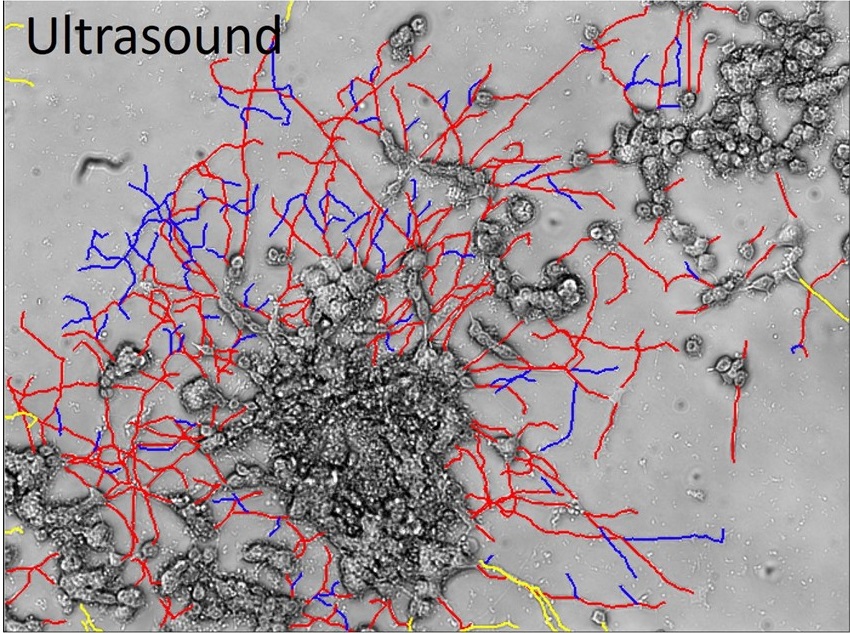
PC-12 cells exposed to pulsed ultrasound at a frequency of 0.68 MHz and intensity of 1.64 W/cm2. Shown are neurites with (red) and without connections (blue).
Research Area 3: Acoustic Tweezing Analysis of Biological Materials
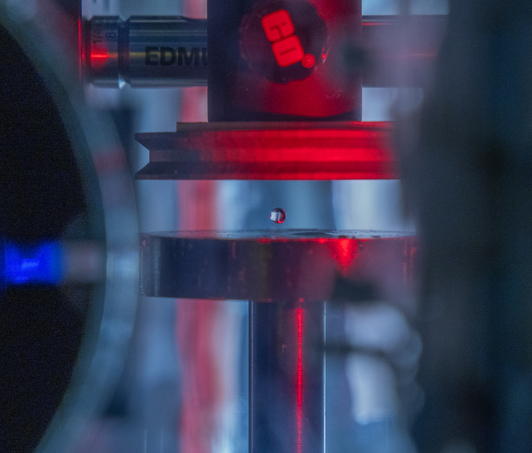
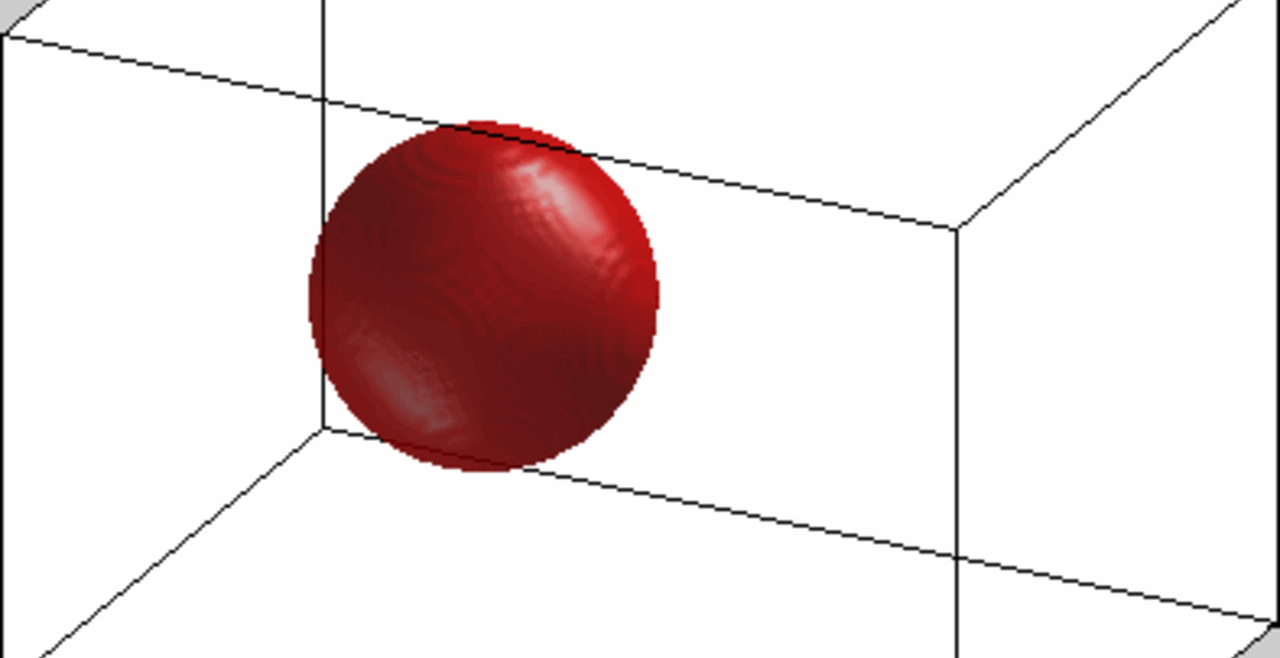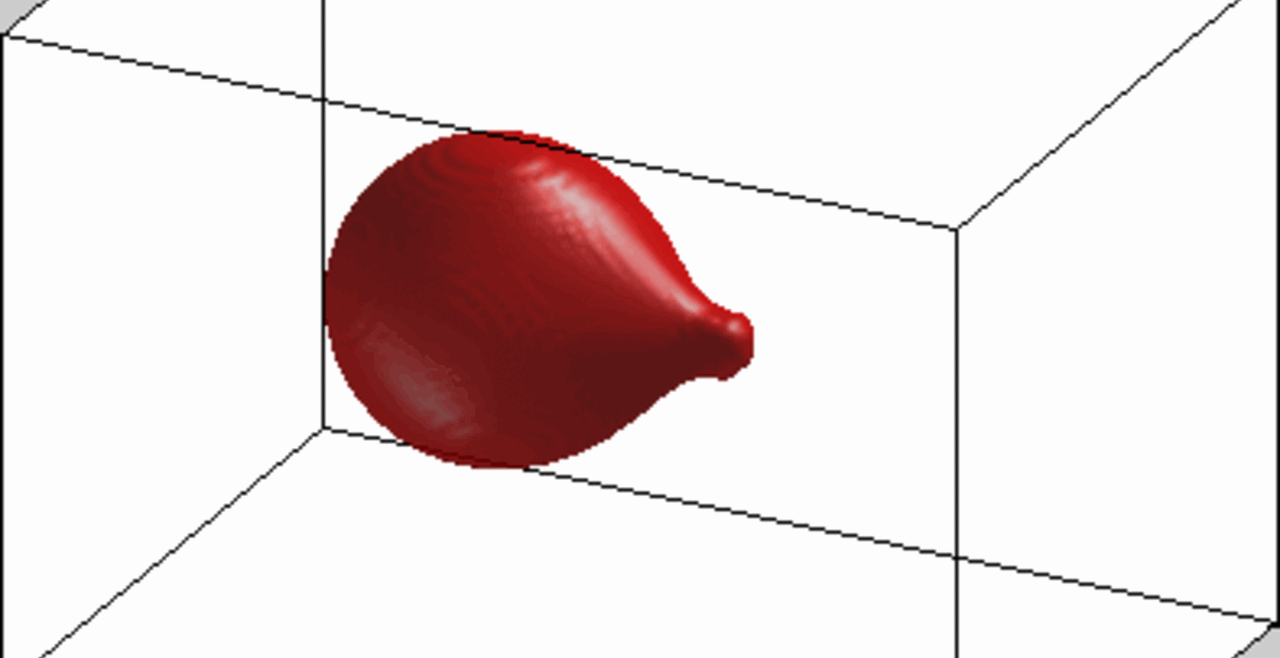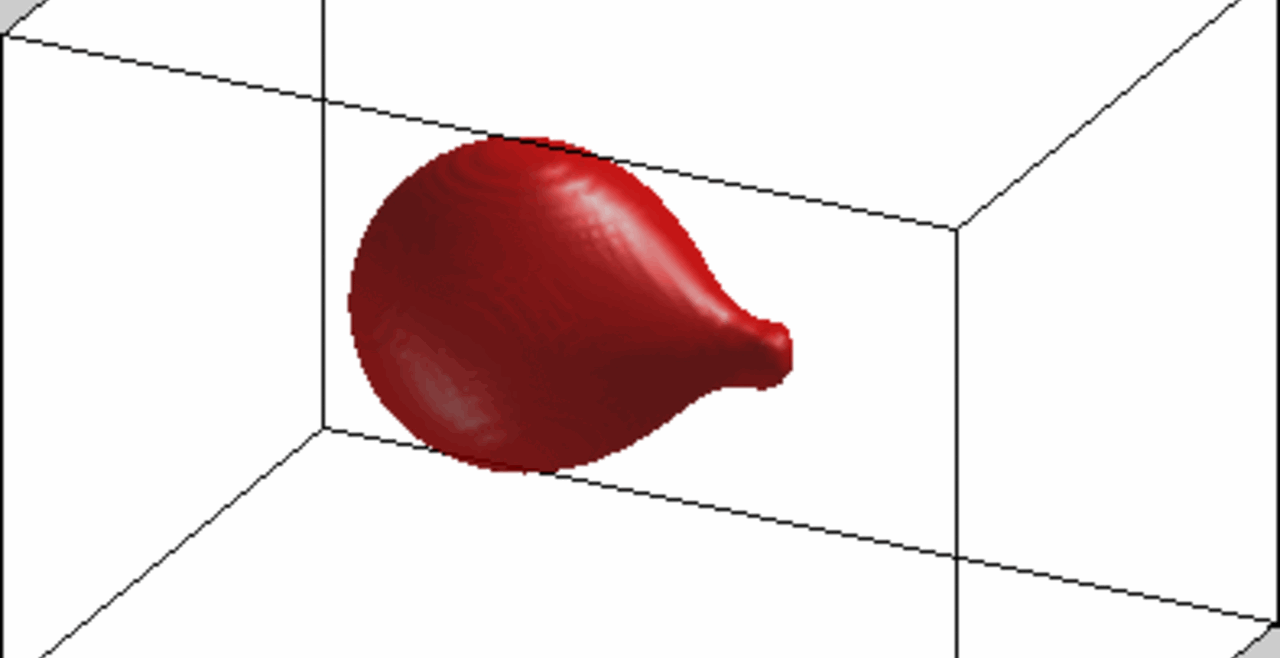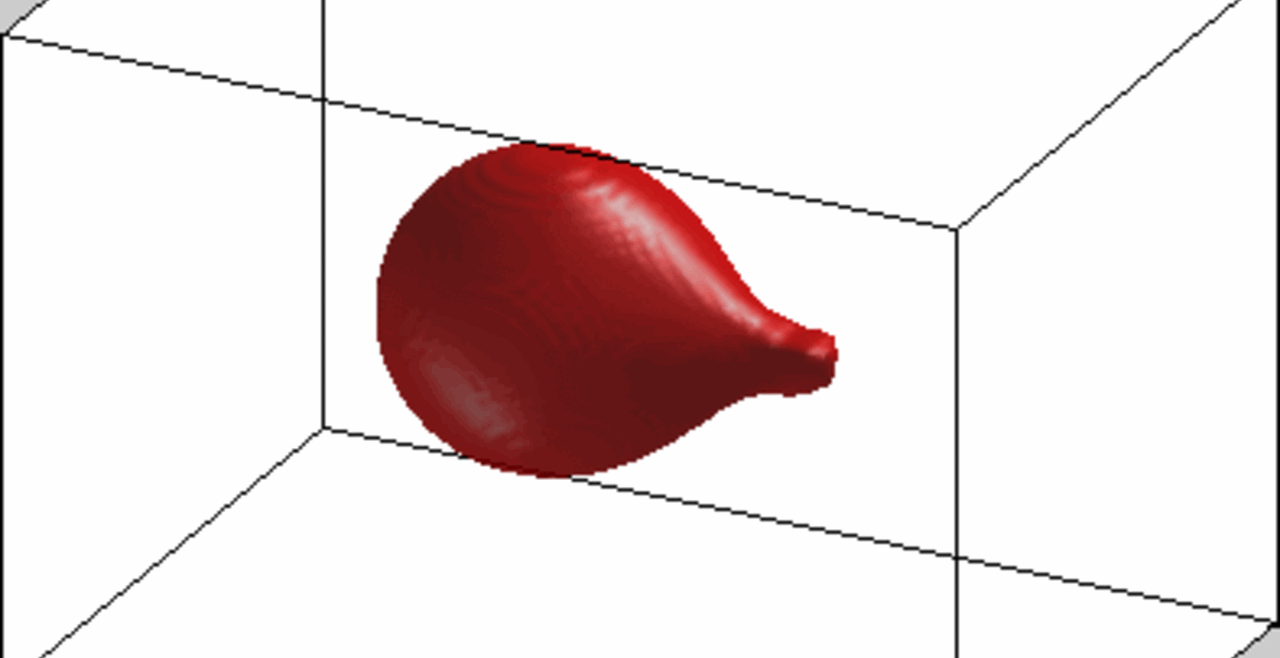
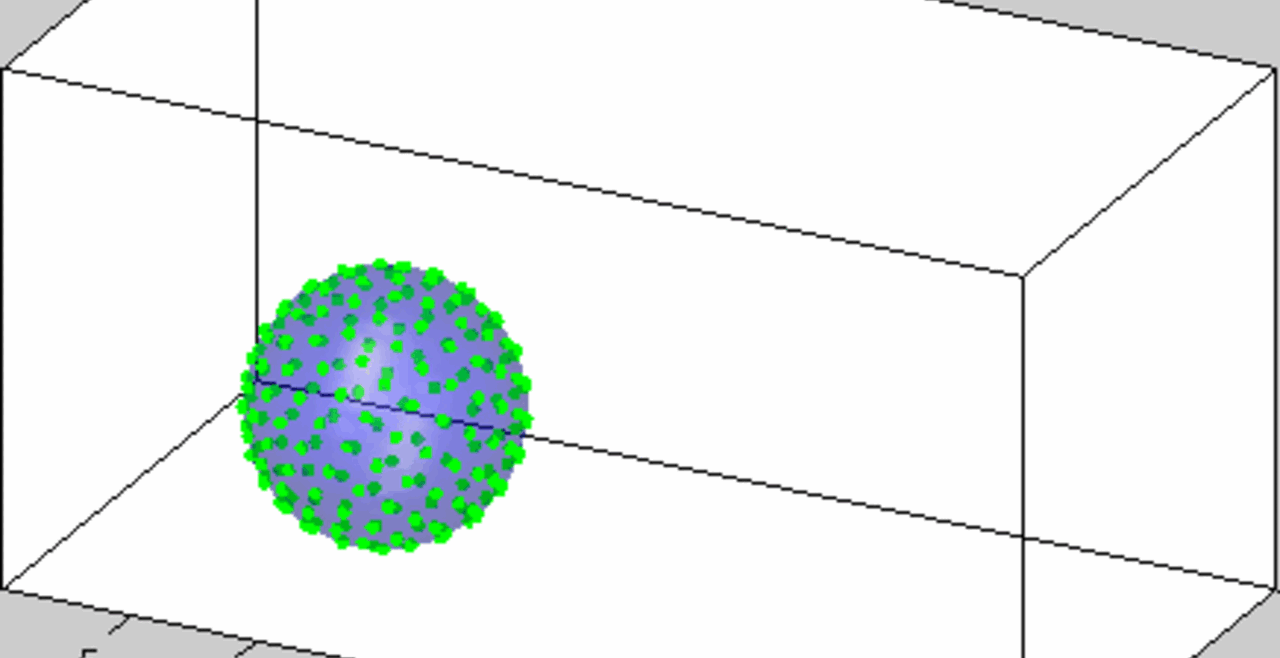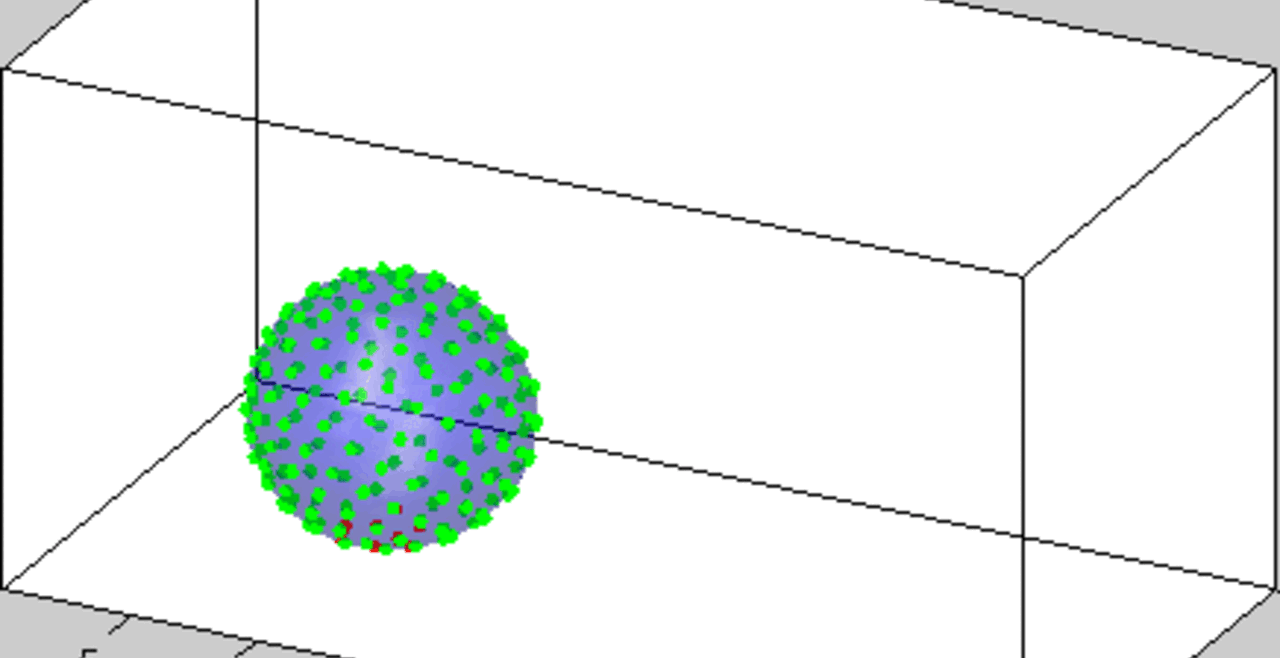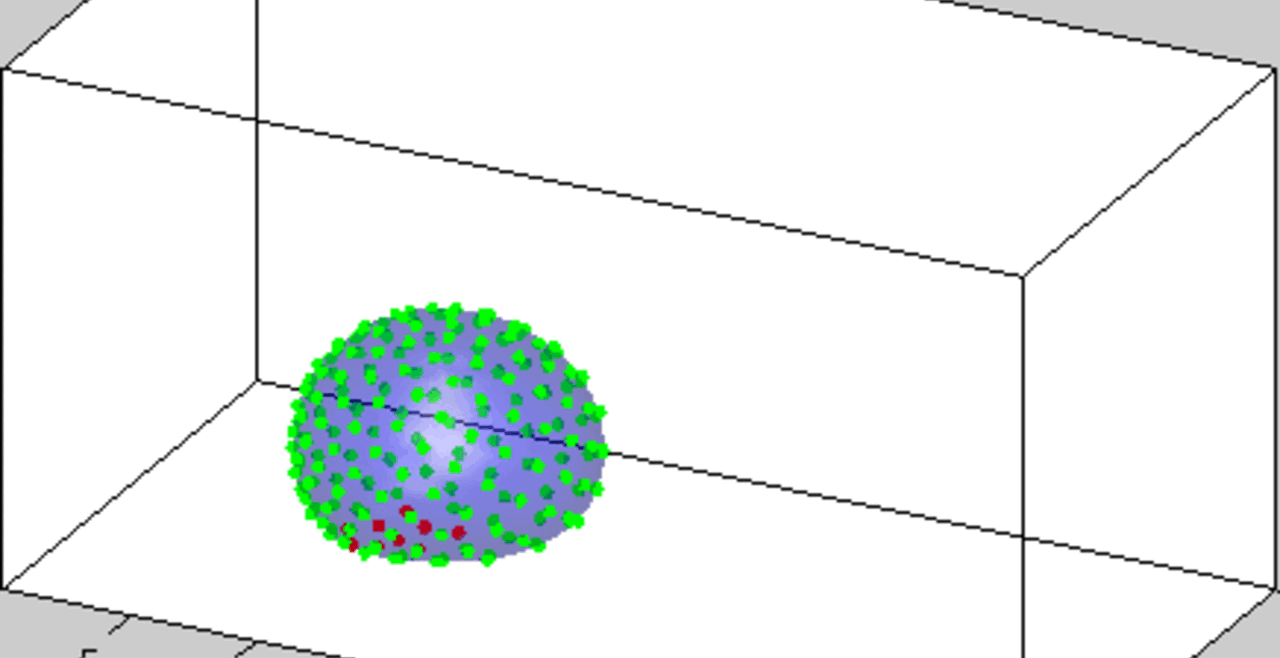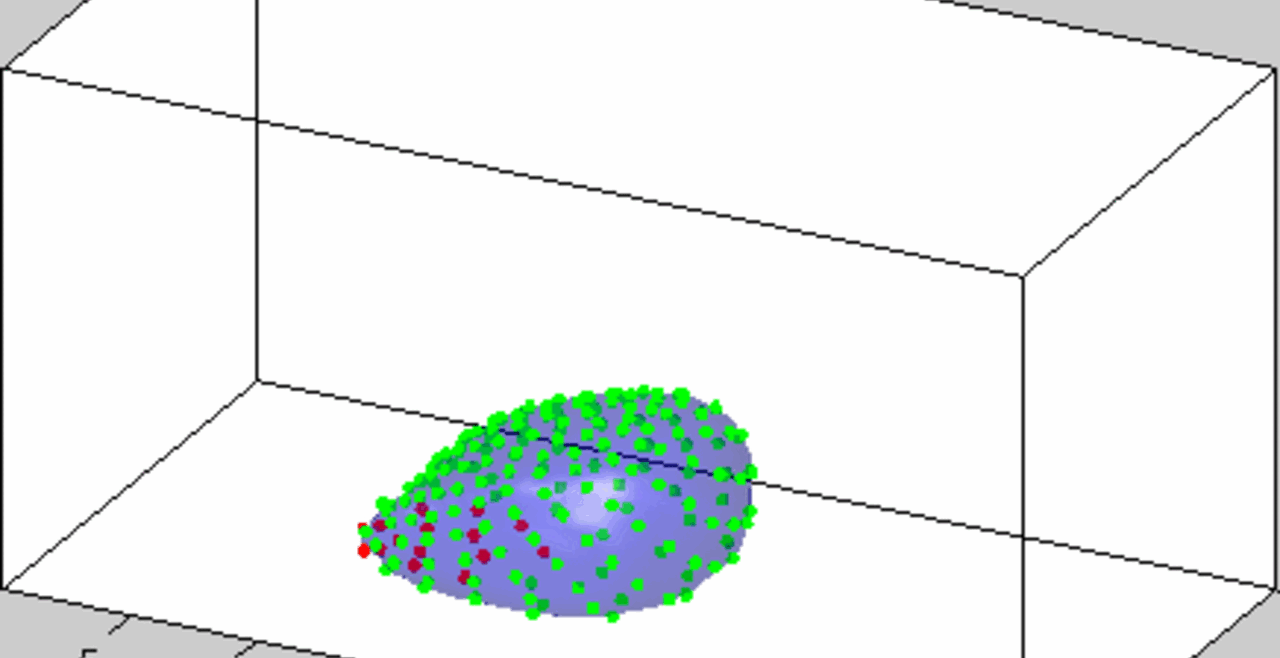
An acoustically levitated drop of a biological sample.
The development of acoustic tweezing technology has been the primary focus of the Biomedical Acoustics laboratory over the past decade. The essence of this technology lies in the acoustic levitation of a single drop of fluid and the measurement of the mechanical response of the levitated drop to changes in the acoustic pressure. This unique, non-contact approach enables temporal rheological and structural analyses of fluids with a sample volume of less than 10 microliters (typically 4-6 microliters). Acoustic tweezing is a platform technology that provides accurate rheological characterization of a wide class of biological and other fluids that can dynamically change their internal structure.
With funding from the American Heart Association and the National Science Foundation, we built a fully functional laboratory prototype of the acoustic tweezing device in 2015 and successfully tested it for measuring the viscosity and elasticity of dextran, gelatin, and alginate solutions, as well as medical viscosity standard fluids. We developed three acoustic tweezing techniques for blood coagulation analysis, collectively referred to as acoustic tweezing coagulometry: (1) quasi-static acoustic tweezing thromboelastometry (QATT), (2) integrated quasi-static acoustic tweezing thromboelastometry (i-QATT), and (3) acoustic tweezing spectroscopy (ATS). Each technique requires only a single 4-6 microliter drop of blood, approximately 100 times less than the sample volume needed in conventional clinical coagulation analyzers.

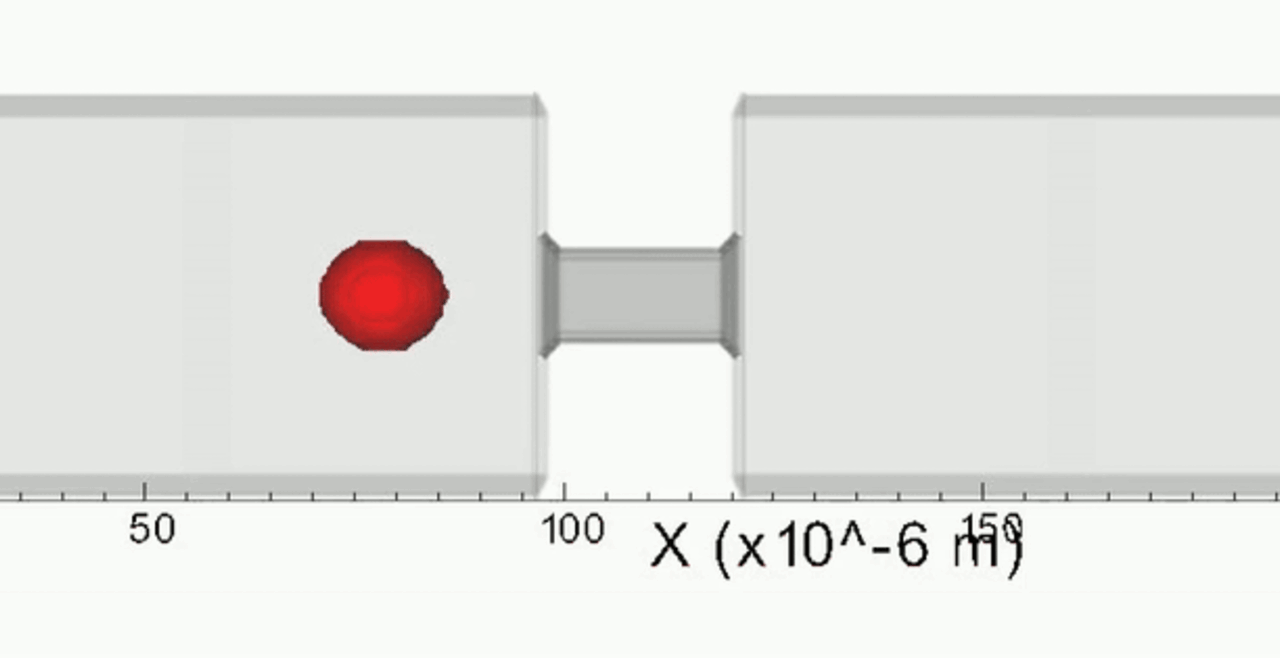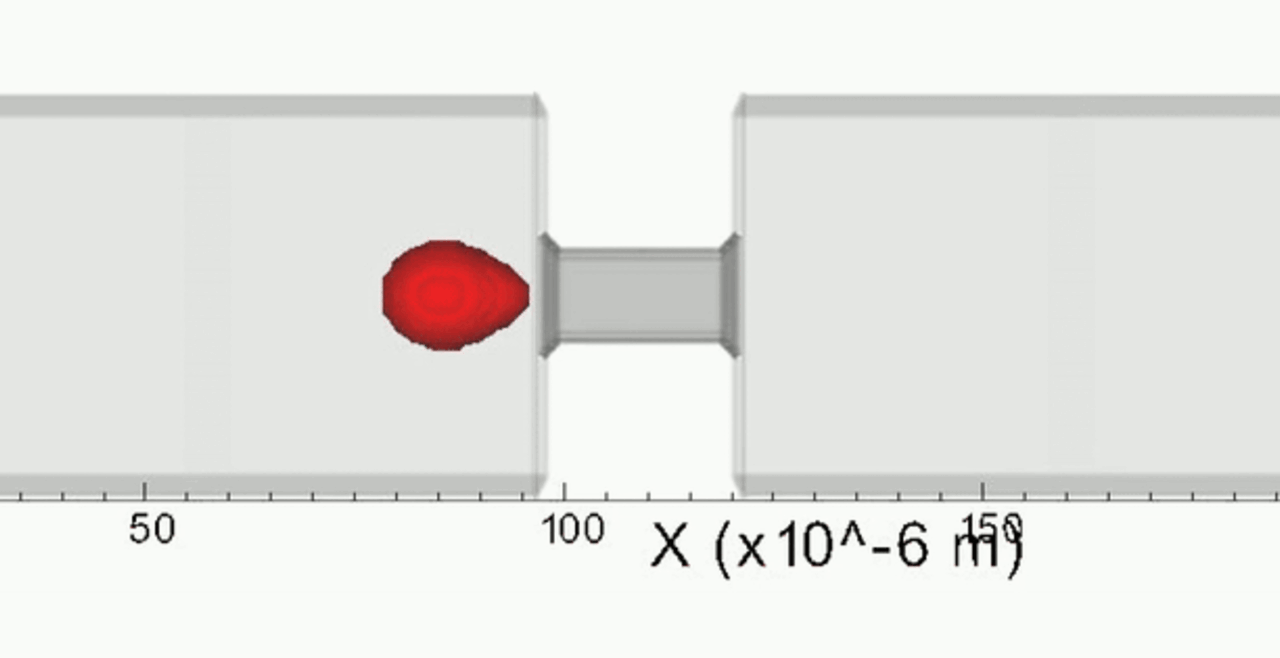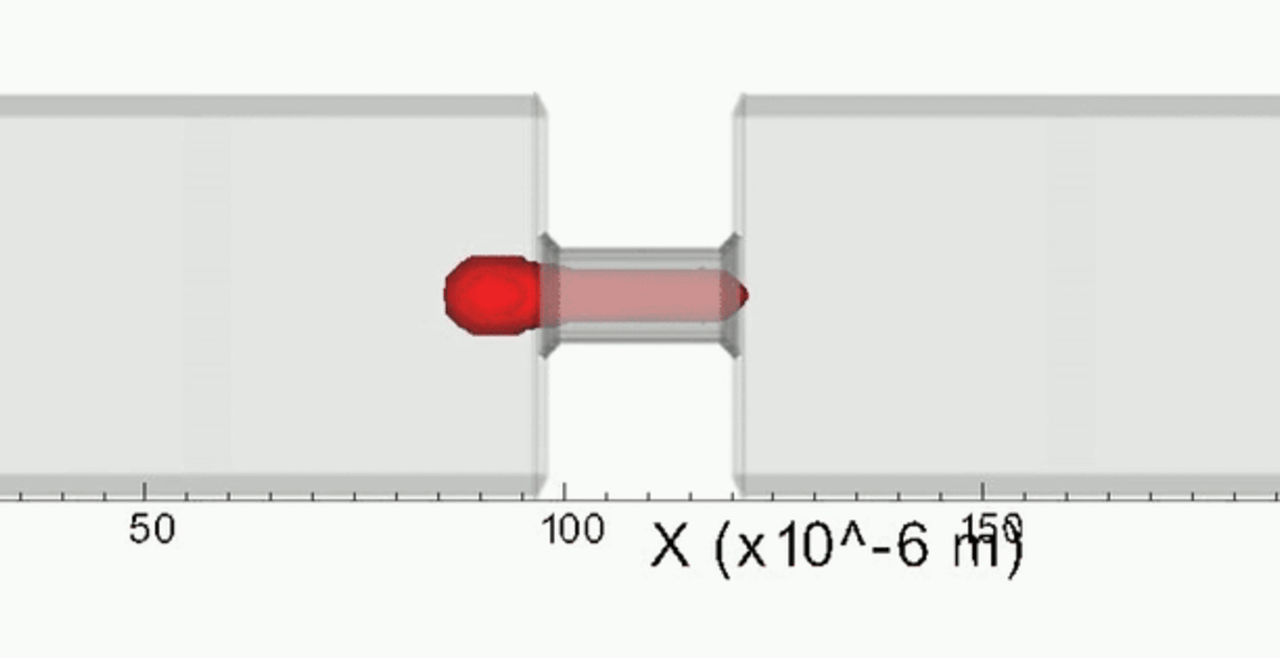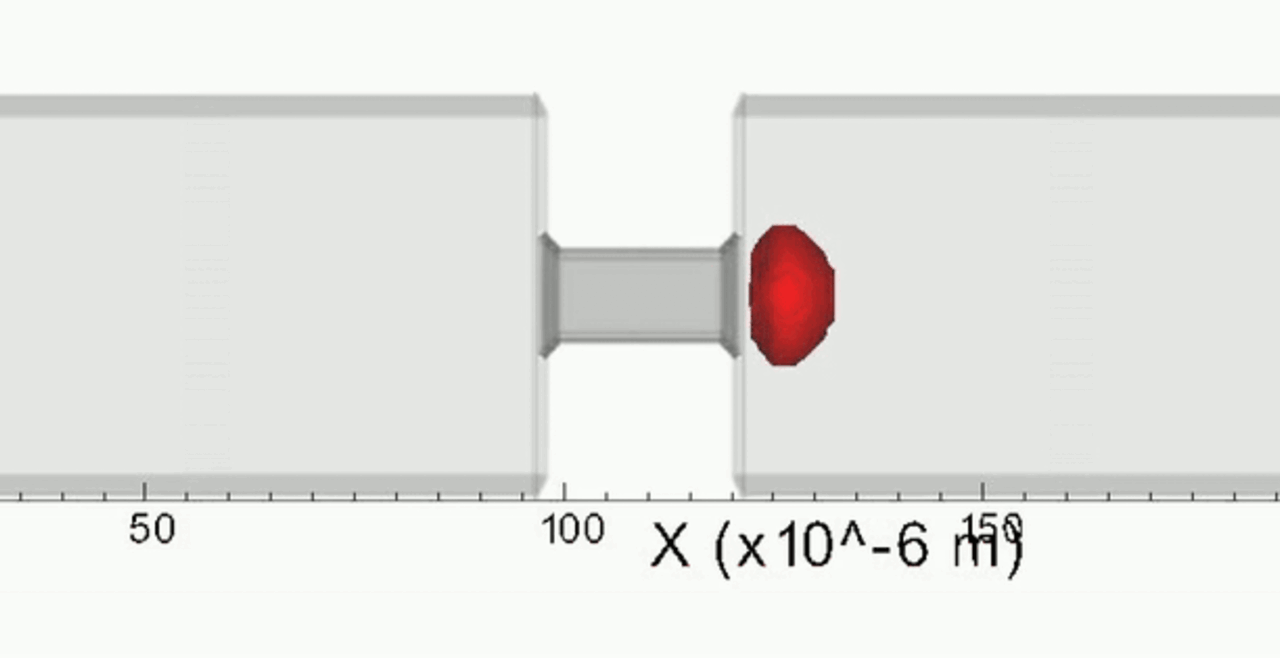
An increase in acoustic pressure lifts a levitated drop of blood to a higher position and increases its aspect ratio. This is the basic principle of the QATT technique. From Holt et al. J. Thromb. Haemost. 15, 1453-1462 (2017).
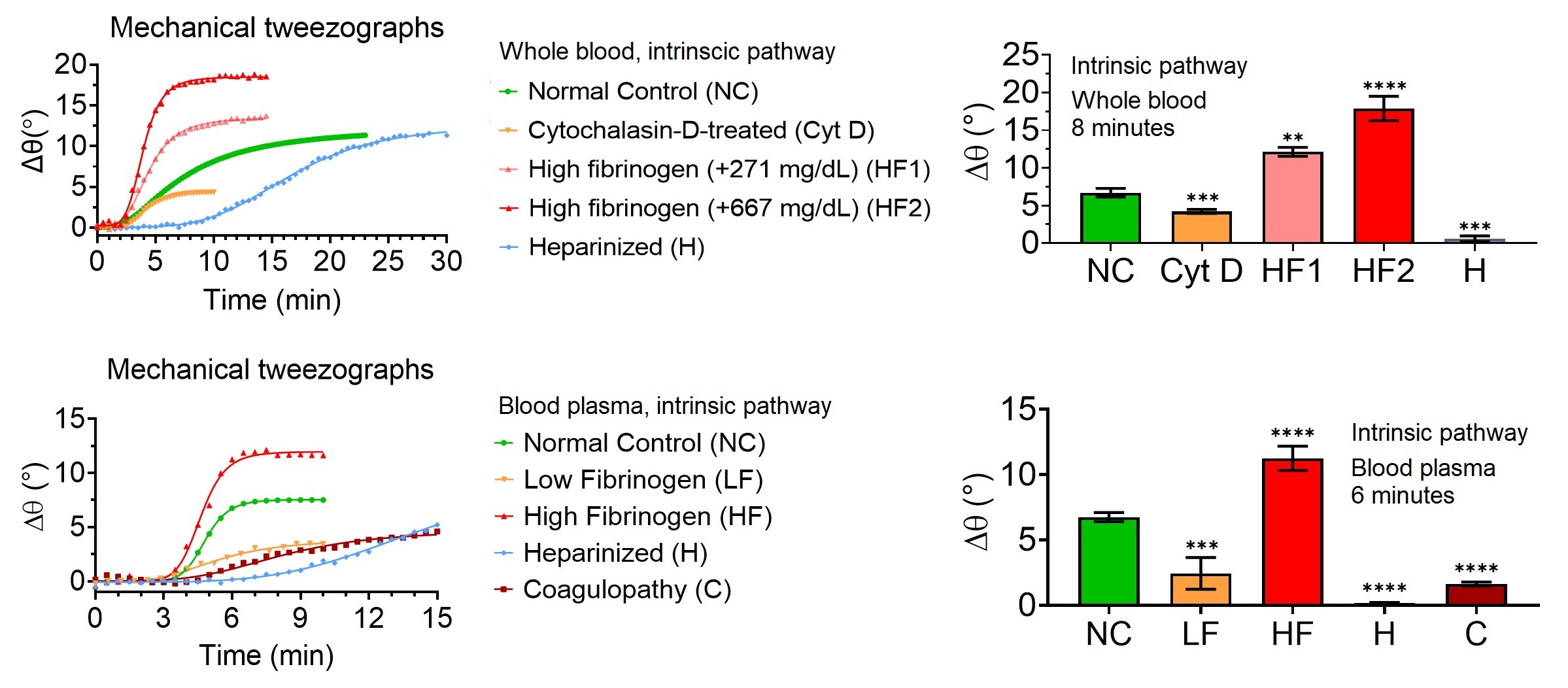
The QATT graphical output (mechanical tweezograph, left) and clot firmness data (right) demonstrate that the technique detects abnormal clotting as early as 8 minutes in whole blood and 6 minutes in plasma samples when coagulation is initiated via the intrinsic pathway. Detection times are even shorter when the extrinsic pathway is used for activation.
The key disruptive features of acoustic tweezing coagulometry include: (1) a very small sample volume requirement, potentially eliminating the need for venipuncture; (2) improved reliability and accuracy through non-contact measurement; (3) a short procedure time, with coagulation defects detected in under 6 minutes in plasma and 8 minutes in whole blood; and (4) comprehensive coagulation analysis that integrates both viscoelastic and photo-optical measurements.
Our experiments with human blood samples demonstrated the ability of this technology to measure fibrinogen concentration, platelet count and activity, hematocrit, doses of heparin and other anticoagulants, fibrinolysis, and disseminated intravascular coagulation. The acoustic tweezing coagulometry data showed strong linear correlations with gold-standard plasma-based coagulation tests (PT, INR, aPTT) and whole-blood viscoelastic hemostatic assays (TEG, ROTEM). We also demonstrated its ability to measure fibrin kinetics (including PT and aPTT) in whole blood or in dark, high-hemoglobin plasma (hemolyzed samples) that are typically unusable for conventional photo-optical turbidimetric analysis due to the blockage of the optical signal by hemoglobin.
In 2016, we founded Levisonics Inc. to commercialize acoustic tweezing coagulometry. To date, both Levisonics and our laboratory have secured over $7.5 million in nondilutive funding from major agencies (NIH, NSF, DoD, AHA) to advance this technology for coagulation analysis in pediatric and adult patients with coagulation or cardiovascular disorders, as well as for patients undergoing major surgery or receiving critical care. Most of these projects are ongoing.

The commercial prototype of the acoustic tweezing device features a compact, shoebox-sized footprint.

Funding
 Our research has been funded by grants from the National Science Foundation, the National Institutes of Health, the Department of Defense, the American Heart Association, and the Louisiana Board of Regents.
Our research has been funded by grants from the National Science Foundation, the National Institutes of Health, the Department of Defense, the American Heart Association, and the Louisiana Board of Regents.