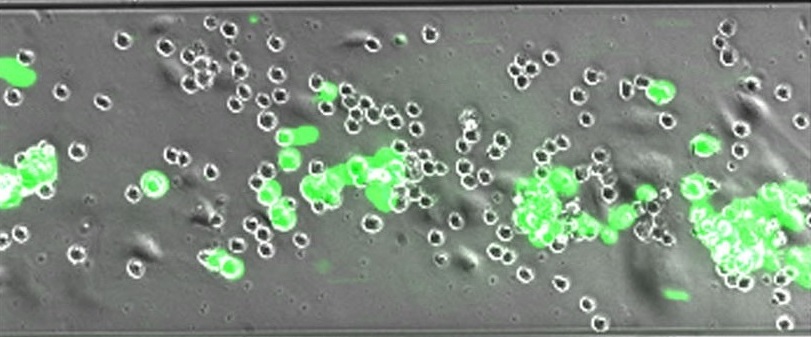
We carry out experimental and theoretical research in three major areas: 1) biomechanics of myeloid, endothelial, and cancer cells; 2) therapeutic ultrasound; and 3) acoustic tweezing analysis of biological materials. In the first area, we aim to uncover the underlying causes of atherosclerosis, cancer, and neurodegenerative diseases. To achieve this, we study phenotypic changes in and interactions between human monocytes/macrophages, mast cells, vascular endothelial cells, and cancer cells under various pathophysiological conditions. This work employs advanced microscopy, flow cytometry, deformability cytometry, cytokine and proteomic analyses, microfluidic flow adhesion and migration assays, and state-of-the-art in silico modeling.
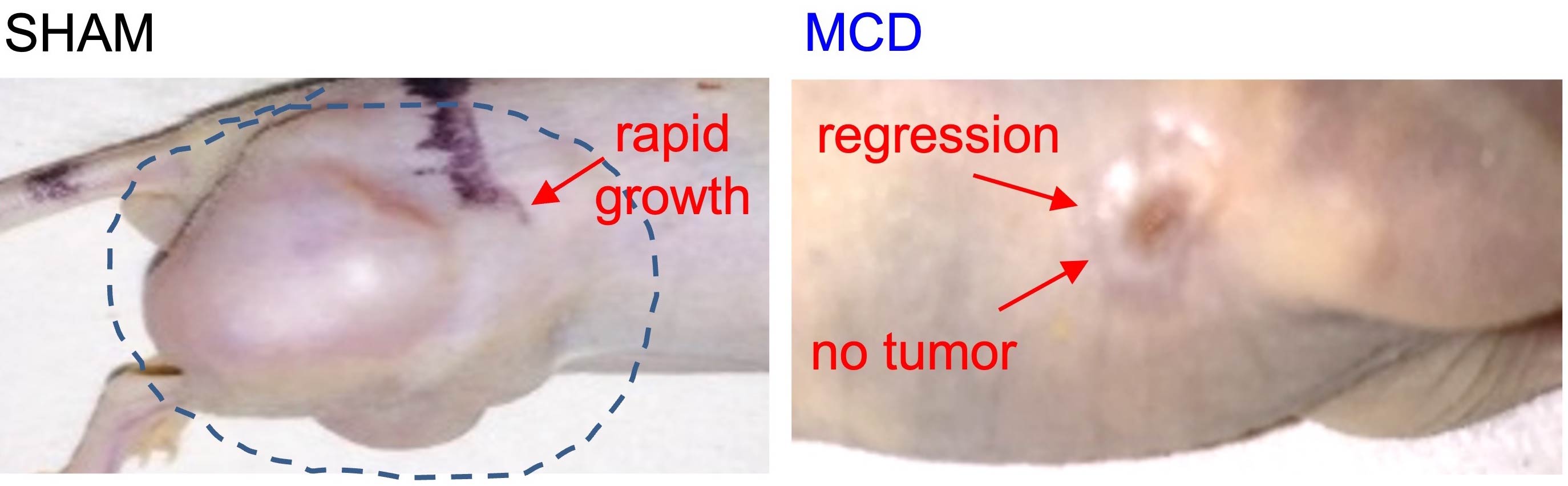
In the second area, we investigate how living cells, tissues, and biological polymers respond to mechanical forces generated by acoustic waves. In particular, we explore the potential of combining focused ultrasound with specific chemical agents to induce cellular reprogramming. This innovative approach, referred to as "mechanochemical reprogramming", holds promise for applications such as nerve regeneration (e.g., in spinal cord injury and vision loss), cancer treatment, and genetic engineering/biotechnology. Our recent data demonstrate the complete regression of tumors in vivo post mechanochemical disruption (MCD) induced by focused ultrasound and ethanol, one of the histone acetylation agents.

In the third area, we focus on developing novel acoustics-based methods for material characterization and medical diagnostics. We have patented "acoustic tweezing rheometry", a technology that measures material properties and their changes over time using a small, levitated drop of sample. Both our lab and our spinoff company, Levisonics Inc., currently translate the acoustic tweezing technology into clinical applications, such as blood coagulation analysis. Specifically, we investigate its use for monitoring and managing patients with hemophilia and sickle cell disease, those in critical care undergoing ECMO or hemodialysis, trauma and surgical patients requiring transfusions, cardiovascular patients on anticoagulant therapy, and patients with infectious diseases.
Lab updates
In March of 2025, Dr. Georgy Sankin joined us as a Senior Research Scientist. He came from the Duke University School of Engineering. His research is supported by the DoD grant and focuses on the application of acoustic tweezing spectroscopy to monitoring and management of trauma-induced coagulopathy.
News articles
Tulane Today has published an article about our acoustic tweezing technology and its application to the management of trauma-induced coagulopathy. The Department of Defense supports the development of this clinical application.
Our Collaborators